Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (7): 2206-2223.doi: 10.19799/j.cnki.2095-4239.2024.0376
• Special Issue on Low Temperature Batteries • Previous Articles Next Articles
Lifeng WANG( ), Naiqing REN, Hai YANG, Yu YAO, Yan YU(
), Naiqing REN, Hai YANG, Yu YAO, Yan YU( )
)
Received:2024-04-28
Revised:2024-06-08
Online:2024-07-28
Published:2024-07-23
Contact:
Yan YU
E-mail:wlifeng@mail.ustc.edu.cn;yanyumse@ustc.edu.cn
CLC Number:
Lifeng WANG, Naiqing REN, Hai YANG, Yu YAO, Yan YU. Advances in low-temperature electrolytes for sodium-ion batteries[J]. Energy Storage Science and Technology, 2024, 13(7): 2206-2223.
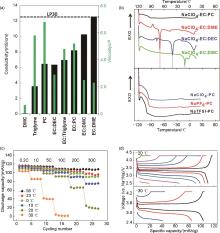
Fig. 2
(a) Conductivity (left hand side y axis) and viscosity (right hand side y axis) values of electrolytes based on 1 mol/L NaClO4 dissolved in various solvents and solvent mixtures; (b) DSC heating curves of electrolytes after cooling the sample down to -120 ℃. Electrolytes based on 1 mol/L NaClO4 dissolved in various solvent mixtures (top) and PC based electrolytes with 1 mol/L of various Na salts (bottom)[41]; (c) Rate performance of the NVP@C at different temperatures; (d) charge/discharge profiles of the NVP@C electrodes with different rates at -20 ℃ to -30 ℃[42]"
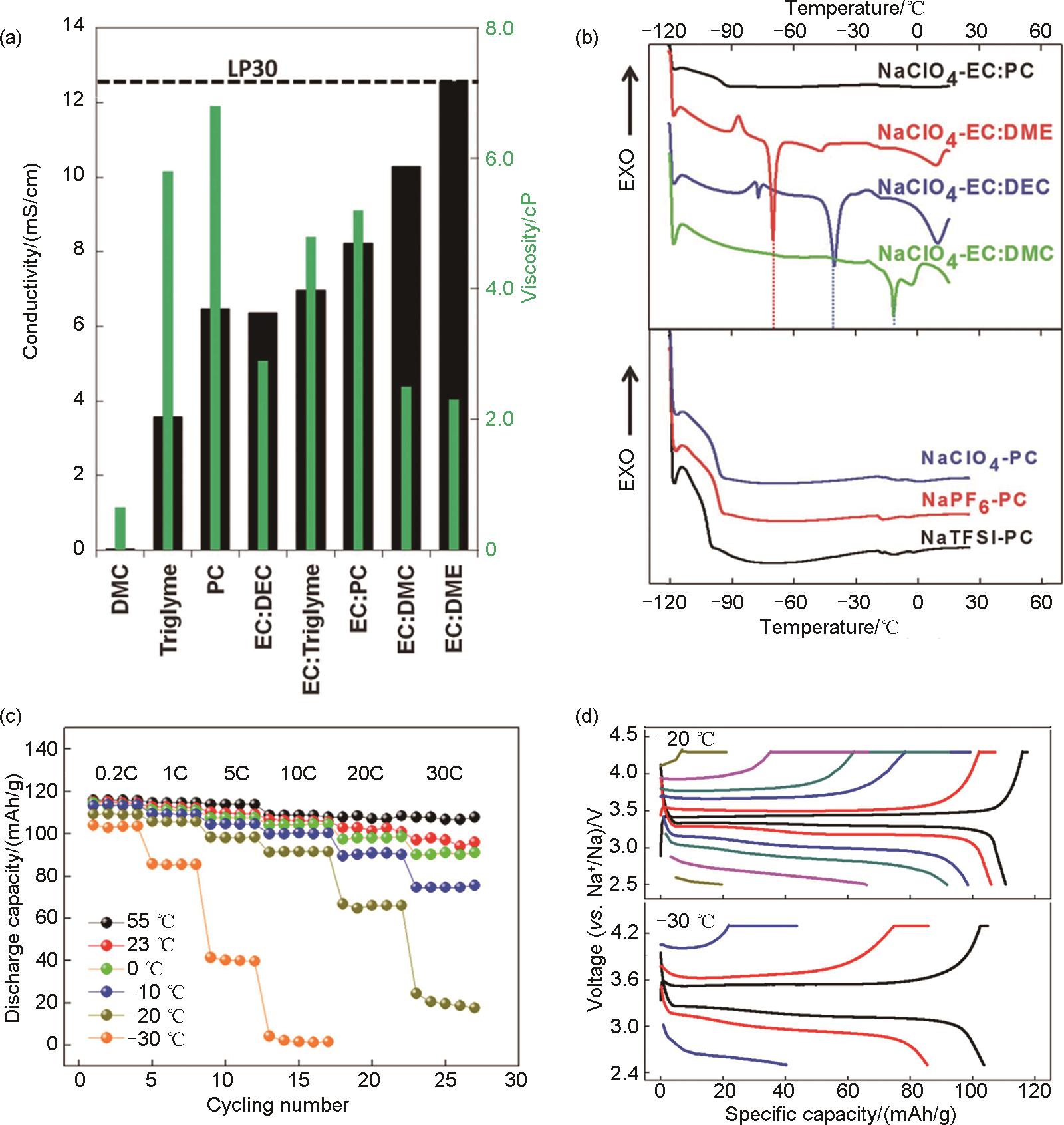
Fig. 3
(a) Concentration dependence of ionic conductivity and viscosity at 25 and 0 ℃ for NaPF6 in EC/PC electrolyte[43]; (b), (c) The solvation structure computed by Ab initio molecular dynamics (AIMD) simulations, which are displayed as snapshots based on NaClO4 dissolved in a EC-PC-FEC system; Most probable solvation structure extracted from AIMD simulations together with their binding energy values (B.E.): (d) the weakly-solvating 0.3 mol/L electrolyte (labeled as WSE), (e) the normal-used standard 1.0 mol/L electrolyte (labeled as NUE); (f), (g) TEM images of the NVPF cathodes after cycling; (h) Long-term cycling performance of the NVPF cathodes at 1C at -25 ℃[44]"
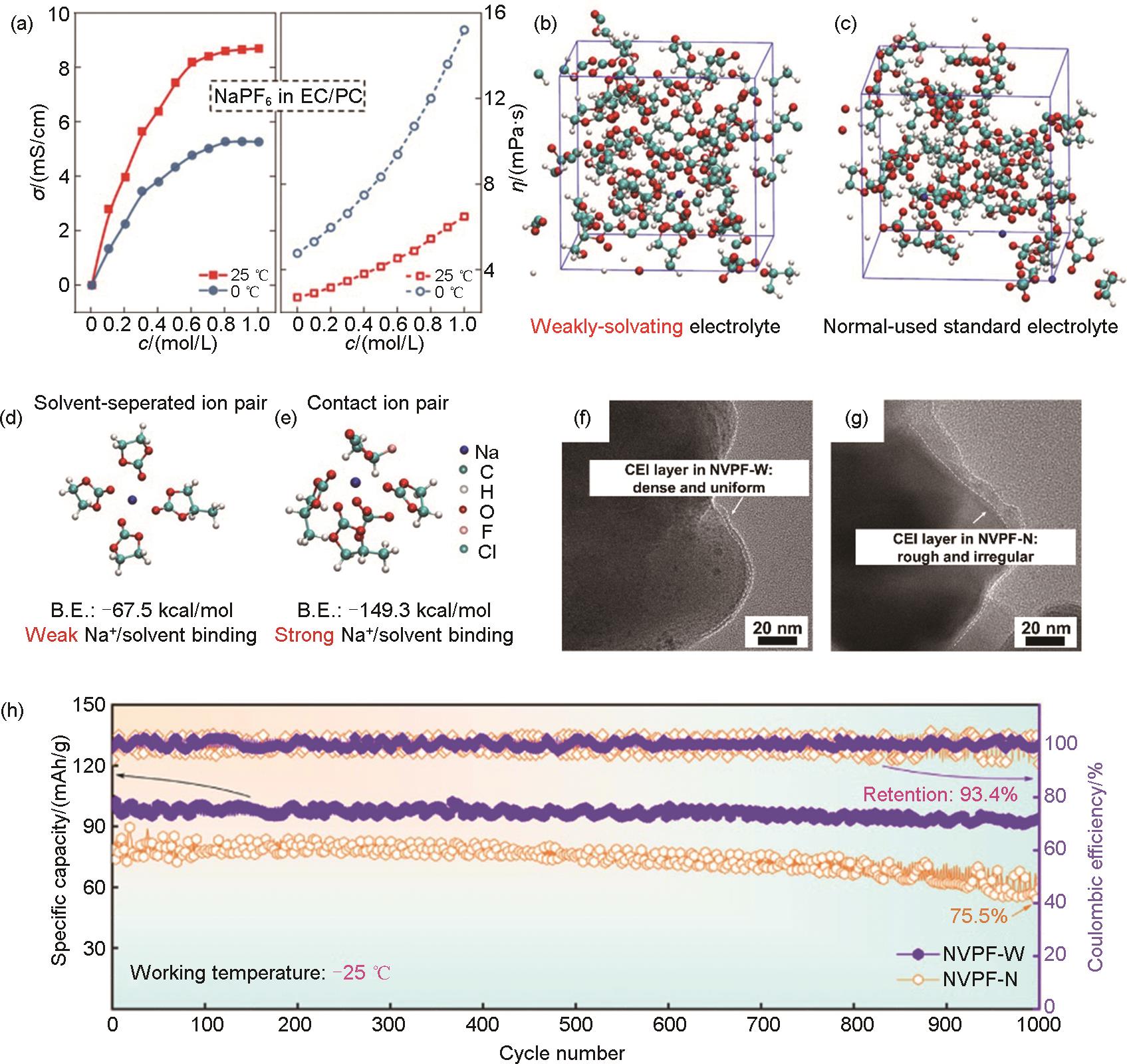

Fig. 4
(a) Representative solvation sheath determined by MD simulations, (b) Viscosity and (c) ionic conductivity test of the carbonate, F-carbonate and WT electrolytes under different temperatures; (d) The binding energy between Na+ ions and corresponding solvents; (e) Impedance spectra the Na||Na symmetric cells with the WT and carbonate (inset image) electrolytes at -20~60 ℃ after 3 formation cycles; (f) Long-term cycling performance of Na||NVP cells operated in the corresponding electrolytes at 0.5C, -20 ℃[51]"
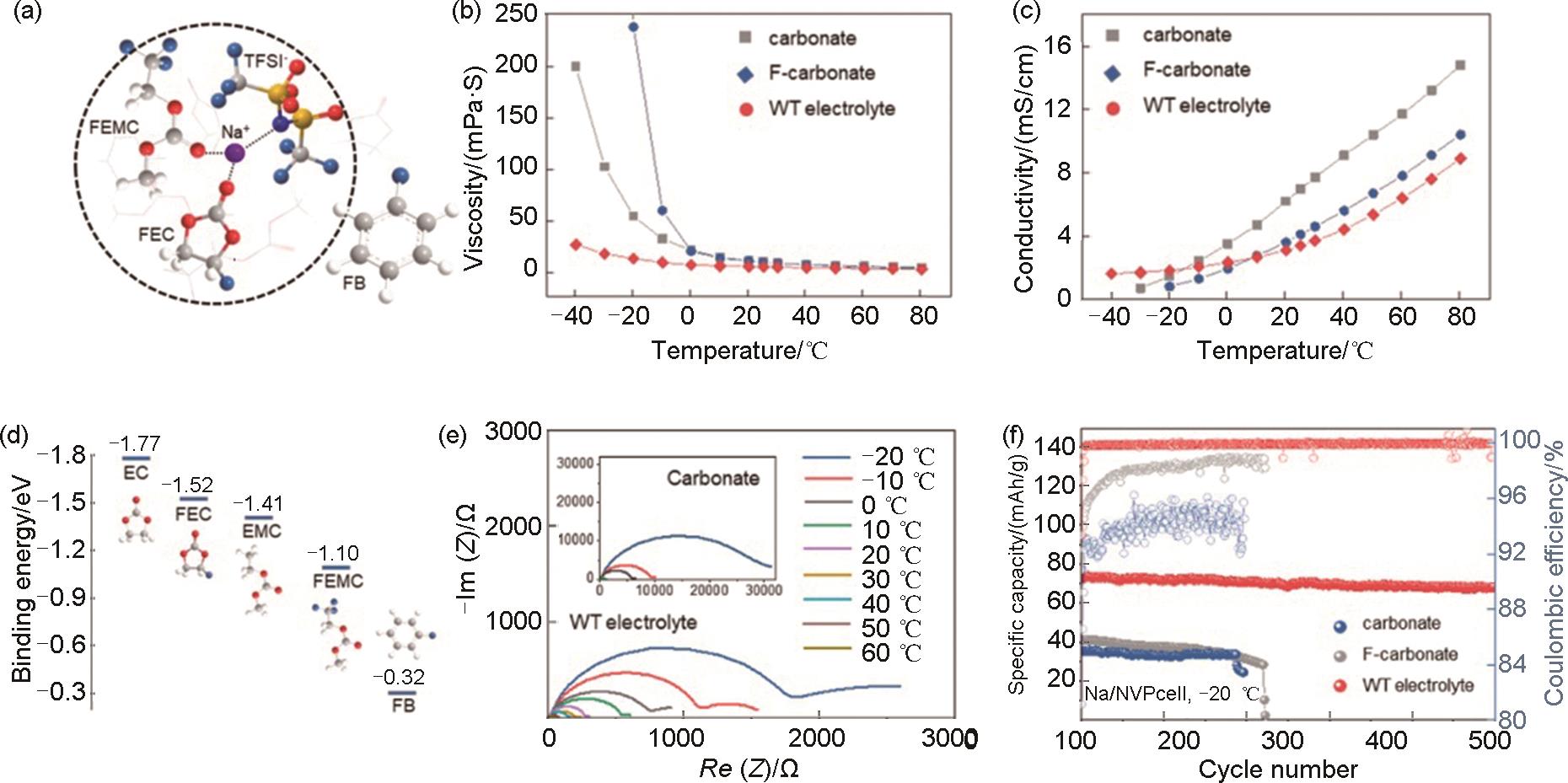
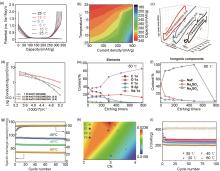
Fig. 5
(a) Galvanostatic charge and discharge curves of HCP at 50 mA/g at the temperature range from -25 to 25 ℃; (b) Temperature-dependent capacity retention at various current densities[52]; (c) DSC thermograms of different electrolytes from 0 °C to -150 ℃; (d) Temperature-dependent ionic conductivity of the electrolyte solutions in the range between 20 ℃ and -80 ℃; (e) Contents of C 1s, O 1s, F 1s, S 2p and Na 1s elements in the SEI at -80 ℃; (f) Contents of the main SEI inorganic components, including NaF, Na2SO4 and Na2SO3, at -80 ℃; (g) Long-term cycling performance of cells at 22 mA/g at -20 ℃, -40 ℃ and -60 ℃[27]; (h) Calculated electron density of BCP for Na+ —O bond in three electrolytes. (I) O in N-THF, (II) O of THF in N-mixTHF, (III) O in N-2MeTHF, and (IV) O of 2MeTHF in N-mixTHF; (i) Long cycling performance in N-mixTHF at different temperatures (100 mA/g beyond -20 ℃, 50 mA/g at -40 ℃, and -60 ℃)[54]"
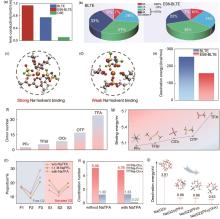
Fig. 6
(a) Ionic conductivity of the CSE, BLTE and ES6-BLTE electrolytes at -40 ℃; (b) The numbers of FSI- around Na+ in the solvated structure obtained from the MD simulations in BLTE and ES6-BLTE (A represents the number of FSI- coordinated with per Na+. In the SSIPs structure, Na+ is only coordinated with solvent in the first solvation shell; in the CIPs structure, Na+ is coordinated with one FSI-; in the AGGs structure, Na+ is coordinated with more than one FSI-); Most probable solvation structure extracted from MD simulation for (c) BLTE and (d) ES6-BLTE electrolytes; (e) The desolvation energy of BLTE and ES6-BLTE electrolytes obtained from DFT calculations[61]; (f) Donor number of commonly used anions; (g) Binding energy of Na+ with anions; (h) Proportion of free G2 and solvated G2 in different electrolytes; (i) Coordination number from MD simulation of electrolytes without and with NaTFA; (j) Desolvation energy of four representative solvation configurations[33]"
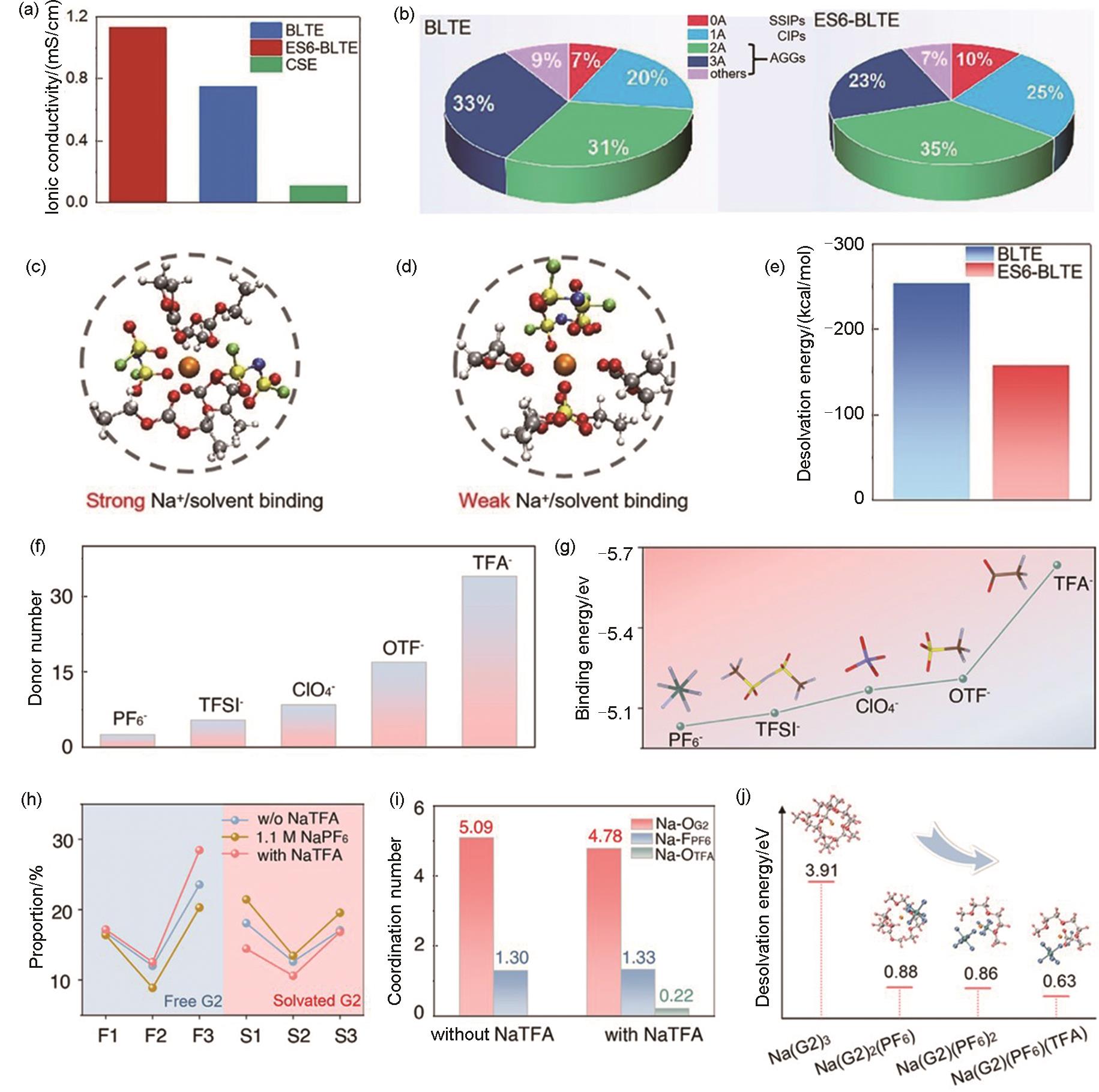
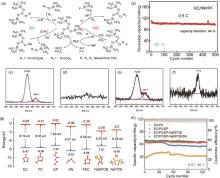
Fig. 7
(a) Diagram of the synergistic regulatory mechanisms driven by distinct intermediates for interfacial modification and HF consumption with the role played by TMSPi and FEC; (b) Cycling performances with the NPFT electrolyte at -25 ℃[26]; XPS spectra of the cathode in the battery with the electrolyte containing 0% ADN after the first charge-discharge cycle: (c) F 1s and (d) N 1s; XPS spectra of the cathode in the battery with the electrolyte containing 3% ADN after the first charge-discharge cycle: (e) F 1s and (f) N 1s[67]; (g) Calculated lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) energy levels for the solvent molecules, sodium salts, additives; (h) The capacity retention of Na||NVP cells using varied electrolytes at 0.1C under -45 ℃[35]"
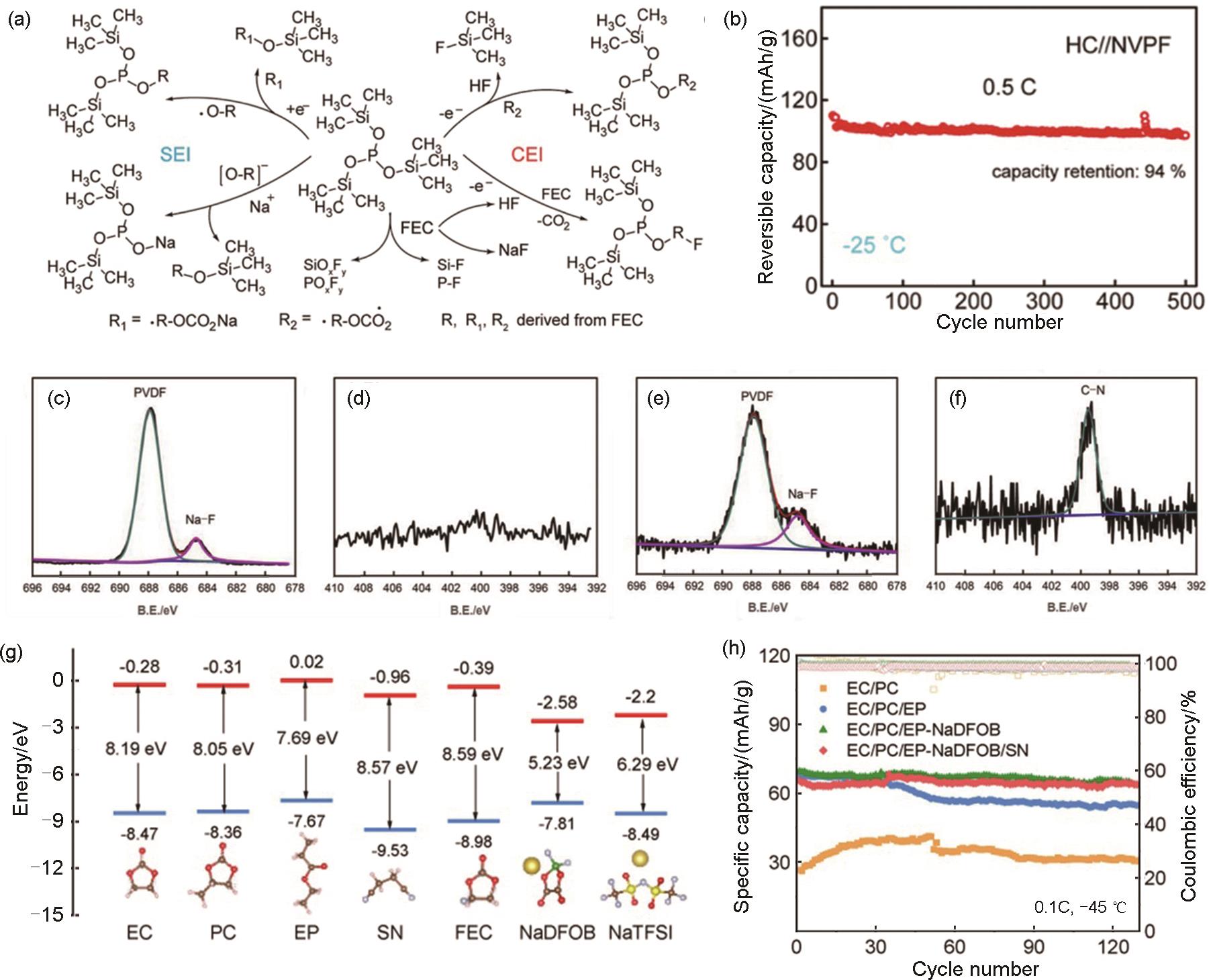
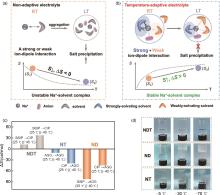
Fig. 8
The solvation structure variations with temperature driven by entropy change: (a) non adaptive electrolyte; (b) temperature-adaptive electrolyte. ΔS here, is the difference between the solvation entropy at room temperature (S1) and low temperature (S2), i.e., ΔS = S2-S1. RT: room temperature; LT: low temperature; (c) The calculated entropy change values along with solvation structure variations. The ∆ S of solvation structure change from 25 ℃ to -40 ℃ shows a positive value at NDT system, while it keeps a negative entropy change for ND and NT; (d) Photographs of NDT, ND, and NT electrolytes stored at different temperatures[72]"

| 1 | MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry[J]. Nature Communications, 2020, 11(1): 1550. DOI: 10.1038/s41467-020-15355-0. |
| 2 | CHEN S Q, SHEN L F, VAN AKEN P A, et al. Dual-functionalized double carbon shells coated silicon nanoparticles for high performance lithium-ion batteries[J]. Advanced Materials, 2017, 29(21): 1605650. DOI: 10.1002/adma.201605650. |
| 3 | ZHU C B, SONG K P, VAN AKEN P A, et al. Carbon-coated Na3V2(PO4)3 embedded in porous carbon matrix: An ultrafast Na-storage cathode with the potential of outperforming Li cathodes[J]. Nano Letters, 2014, 14(4): 2175-2180. DOI: 10.1021/nl500548a. |
| 4 | LI W H, ZENG L C, WU Y, et al. Nanostructured electrode materials for lithium-ion and sodium-ion batteries via electrospinning[J]. Science China Materials, 2016, 59(4): 287-321. DOI: 10.1007/s40843-016-5039-6. |
| 5 | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices[J]. Science, 2011, 334(6058): 928-935. DOI: 10.1126/science.1212741. |
| 6 | THACKERAY M M, WOLVERTON C, ISAACS E D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries[J]. Energy & Environmental Science, 2012, 5(7): 7854-7863. DOI: 10.1039/C2EE21892E. |
| 7 | MACKANIC D G, CHANG T H, HUANG Z J, et al. Stretchable electrochemical energy storage devices[J]. Chemical Society Reviews, 2020, 49(13): 4466-4495. DOI: 10.1039/d0cs00035c. |
| 8 | ZHU C B, MU X K, VAN AKEN P A, et al. Single-layered ultrasmall nanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium storage[J]. Angewandte Chemie International Edition, 2014, 53(8): 2152-2156. DOI: 10.1002/anie.201308354. |
| 9 | PALOMARES V, SERRAS P, VILLALUENGA I, et al. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems[J]. Energy & Environmental Science, 2012, 5(3): 5884-5901. DOI: 10.1039/C2EE02781J. |
| 10 | KIM H, KIM H, DING Z, et al. Recent progress in electrode materials for sodium-ion batteries[J]. Advanced Energy Materials, 2016, 6(19): 1600943. DOI: 10.1002/aenm.201600943. |
| 11 | HWANG J Y, MYUNG S T, SUN Y K. Sodium-ion batteries: Present and future[J]. Chemical Society Reviews, 2017, 46(12): 3529-3614. DOI: 10.1039/c6cs00776g. |
| 12 | WU C, JIANG Y, KOPOLD P, et al. Peapod-like carbon-encapsulated cobalt chalcogenide nanowires as cycle-stable and high-rate materials for sodium-ion anodes[J]. Advanced Materials, 2016, 28(33): 7276-7283. DOI: 10.1002/adma.201600964. |
| 13 | ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. DOI: 10.1038/451652a. |
| 14 | ZHU G L, WEN K C, LV W Q, et al. Materials insights into low-temperature performances of lithium-ion batteries[J]. Journal of Power Sources, 2015, 300: 29-40. DOI: 10.1016/j.jpowsour.2015.09.056. |
| 15 | CHEN P, WU C Y, WANG Z Y, et al. Synergistically boosting sodium-storage performance of Na3V2(PO4)3 by regulating Na sites and constructing 3D interconnected carbon nanosheet frameworks[J]. ACS Applied Energy Materials, 2022, 5(2): 2542-2552. DOI: 10.1021/acsaem.1c04061. |
| 16 | ZHANG H W, SONG J J, LI J Y, et al. Interlayer-expanded MoS2 nanoflowers vertically aligned on MXene@Dual-phased TiO2 as high-performance anode for sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(14): 16300-16309. DOI: 10.1021/acsami.2c02080. |
| 17 | RUI X H, ZHANG X H, XU S T, et al. A low-temperature sodium-ion full battery: Superb kinetics and cycling stability[J]. Advanced Functional Materials, 2021, 31(11): 2009458. DOI: 10.1002/adfm.202009458. |
| 18 | WANG Y Y, HOU B H, GUO J Z, et al. An ultralong lifespan and low-temperature workable sodium-ion full battery for stationary energy storage[J]. Advanced Energy Materials, 2018, 8(18): 1703252. DOI: 10.1002/aenm.201703252. |
| 19 | XIA Y, QUE L F, YU F D, et al. Tailoring nitrogen terminals on MXene enables fast charging and stable cycling Na-ion batteries at low temperature[J]. Nano-Micro Letters, 2022, 14(1): 143. DOI: 10.1007/s40820-022-00885-7. |
| 20 | HWANG J Y, OH S M, MYUNG S T, et al. Radially aligned hierarchical columnar structure as a cathode material for high energy density sodium-ion batteries[J]. Nature Communications, 2015, 6: 6865. DOI: 10.1038/ncomms7865. |
| 21 | XIA X M, XU S T, TANG F, et al. A multifunctional interphase layer enabling superior sodium-metal batteries under ambient temperature and -40 ℃[J]. Advanced Materials, 2023, 35(11): 2209511. DOI: 10.1002/adma.202209511. |
| 22 | ZHENG J M, CHEN S R, ZHAO W G, et al. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes[J]. ACS Energy Letters, 2018, 3(2): 315-321. DOI: 10.1021/acsenergylett.7b01213. |
| 23 | ZHENG X Y, CAO Z, LUO W, et al. Solvation and interfacial engineering enable -40 ℃ operation of graphite/NCM batteries at energy density over 270 Wh·kg–1[J]. Advanced Materials, 2023, 35(10): 2210115. DOI: 10.1002/adma.202210115. |
| 24 | RUAN J F, LUO S N, WANG S F, et al. Enhancing the whole migration kinetics of Na+ in the anode side for advanced ultralow temperature sodium-ion hybrid capacitor[J]. Advanced Energy Materials, 2023, 13(34): 2301509. DOI: 10.1002/aenm.202301509. |
| 25 | SUN Y, LI J C, ZHOU H S, et al. Wide-temperature-range sodium-metal batteries: From fundamentals and obstacles to optimization[J]. Energy & Environmental Science, 2023, 16(11): 4759-4811. DOI: 10.1039/d3ee02082g. |
| 26 | LIANG H J, LIU H H, ZHAO X X, et al. Electrolyte chemistry toward ultrawide-temperature (-25 to 75 ℃) sodium-ion batteries achieved by phosphorus/silicon-synergistic interphase manipulation[J]. Journal of the American Chemical Society, 2024, 146(11): 7295-7304. DOI: 10.1021/jacs.3c11776. |
| 27 | WANG C L, THENUWARA A C, LUO J M, et al. Extending the low-temperature operation of sodium metal batteries combining linear and cyclic ether-based electrolyte solutions[J]. Nature Communications, 2022, 13(1): 4934. DOI: 10.1038/s41467-022-32606-4. |
| 28 | XU K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries[J]. Chemical Reviews, 2004, 104(10): 4303-4417. DOI: 10.1021/cr030203g. |
| 29 | AL-DAWSARI J N, BESSADOK-JEMAI A, WAZEER I, et al. Fitting of experimental viscosity to temperature data for deep eutectic solvents[J]. Journal of Molecular Liquids, 2020, 310: 113127. DOI: 10.1016/j.molliq.2020.113127. |
| 30 | ZHENG X Y, GU Z Y, FU J, et al. Knocking down the kinetic barriers towards fast-charging and low-temperature sodium metal batteries[J]. Energy & Environmental Science, 2021, 14(9): 4936-4947. DOI: 10.1039/D1EE01404H. |
| 31 | JIN Y, LE P M L, GAO P Y, et al. Low-solvation electrolytes for high-voltage sodium-ion batteries[J]. Nature Energy, 2022, 7: 718-725. DOI: 10.1038/s41560-022-01055-0. |
| 32 | WANG M, WANG Q C, DING X Y, et al. The prospect and challenges of sodium-ion batteries for low-temperature conditions[J]. Interdisciplinary Materials, 2022, 1(3): 373-395. DOI: 10.1002/idm2.12040. |
| 33 | ZHOU X Z, HUANG Y H, WEN B, et al. Regulation of anion-Na+ coordination chemistry in electrolyte solvates for low-temperature sodium-ion batteries[J]. Proceedings of the National Academy of Sciences of the United States of America, 2024, 121(5): e2316914121. DOI: 10.1073/pnas.2316914121. |
| 34 | GUO S H. A perspective on low-temperature electrolytes for sodium-ion batteries[J]. Energy Lab, 2023, 1: (3): 230003. DOI: 10.54227/elab.20230003. |
| 35 | HE M X, ZHU L J, YE G, et al. Tuning the electrolyte and interphasial chemistry for all-climate sodium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(21): 2401051. DOI: 10.1002/anie.202401051. |
| 36 | WANG L F, REN N Q, YAO Y, et al. Designing solid electrolyte interfaces towards homogeneous Na deposition: Theoretical guidelines for electrolyte additives and superior high-rate cycling stability[J]. Angewandte Chemie International Edition, 2023, 62(6): e202214372. DOI: 10.1002/anie.202214372. |
| 37 | LI W H, HU S H, LUO X Y, et al. Confined amorphous red phosphorus in MOF-derived N-doped microporous carbon as a superior anode for sodium-ion battery[J]. Advanced Materials, 2017, 29(16): 1605820. DOI: 10.1002/adma.201605820. |
| 38 | DENG X H, ZHANG S, CHEN C, et al. Rational design of electrolytes operating at low temperatures: Does the co-solvent with a lower melting point correspond to better performance?[J]. Electrochimica Acta, 2022, 415: 140268. DOI: 10.1016/j.electacta.2022.140268. |
| 39 | ZHANG F, HE B J, XIN Y, et al. Emerging chemistry for wide-temperature sodium-ion batteries[J]. Chemical Reviews, 2024, 124(8): 4778-4821. DOI: 10.1021/acs.chemrev.3c00728. |
| 40 | GUO J Z, WANG P F, WU X L, et al. High-energy/power and low-temperature cathode for sodium-ion batteries: in situ XRD study and superior full-cell performance[J]. Advanced Materials, 2017, 29(33): 1701968. DOI: 10.1002/adma.201701968. |
| 41 | PONROUCH A, MARCHANTE E, COURTY M, et al. In search of an optimized electrolyte for Na-ion batteries[J]. Energy & Environmental Science, 2012, 5(9): 8572-8583. DOI: 10.1039/C2EE22258B. |
| 42 | LIU T F, WANG B, GU X X, et al. All-climate sodium ion batteries based on the NASICON electrode materials[J]. Nano Energy, 2016, 30: 756-761. DOI: 10.1016/j.nanoen.2016.09.024. |
| 43 | LI Y Q, YANG Y, LU Y X, et al. Ultralow-concentration electrolyte for Na-ion batteries[J]. ACS Energy Letters, 2020, 5(4): 1156-1158. DOI: 10.1021/acsenergylett.0c00337. |
| 44 | DENG L, GOH K, YU F D, et al. Self-optimizing weak solvation effects achieving faster low-temperature charge transfer kinetics for high-voltage Na3V2(PO4)2F3 cathode[J]. Energy Storage Materials, 2022, 44: 82-92. DOI: 10.1016/j.ensm.2021.10.012. |
| 45 | PONROUCH A, PALACÍN M R. On the high and low temperature performances of Na-ion battery materials: Hard carbon as a case study[J]. Electrochemistry Communications, 2015, 54: 51-54. DOI: 10.1016/j.elecom.2015.03.002. |
| 46 | XU Y N, WEI Q L, XU C, et al. Layer-by-layer Na3V2(PO4)3 embedded in reduced graphene oxide as superior rate and ultralong-life sodium-ion battery cathode[J]. Advanced Energy Materials, 2016, 6(14): 1600389. DOI: 10.1002/aenm.201600389. |
| 47 | MA X H, WEI Y Y, WU Y D, et al. High crystalline Na2Ni[Fe(CN)6]particles for a high-stability and low-temperature sodium-ion batteries cathode[J]. Electrochimica Acta, 2019, 297: 392-397. DOI: 10.1016/j.electacta.2018.11.063. |
| 48 | CUI G J, WANG H, YU F P, et al. Scalable synthesis of Na3V2(PO4)3/C with high safety and ultrahigh-rate performance for sodium-ion batteries[J]. Chinese Journal of Chemical Engineering, 2022, 46: 280-286. DOI: 10.1016/j.cjche.2021.06.008. |
| 49 | YOO D J, YANG S, KIM K J, et al. Fluorinated aromatic diluent for high-performance lithium metal batteries[J]. Angewandte Chemie International Edition, 2020, 59(35): 14869-14876. DOI: 10.1002/anie.202003663. |
| 50 | WANG H P, ZHU C L, LIU J D, et al. Formation of NaF-rich solid electrolyte interphase on Na anode through additive-induced anion-enriched structure of Na+ solvation[J]. Angewandte Chemie International Edition, 2022, 61(38): e202208506. DOI: 10.1002/anie.202208506. |
| 51 | LIU X Y, ZHENG X Y, QIN X, et al. Temperature-responsive solid-electrolyte-interphase enabling stable sodium metal batteries in a wide temperature range[J]. Nano Energy, 2022, 103: 107746. DOI: 10.1016/j.nanoen.2022.107746. |
| 52 | HOU B H, WANG Y Y, NING Q L, et al. Self-supporting, flexible, additive-free, and scalable hard carbon paper self-interwoven by 1D microbelts: Superb room/low-temperature sodium storage and working mechanism[J]. Advanced Materials, 2019, 31(40): e1903125. DOI: 10.1002/adma.201903125. |
| 53 | ZHOU J, WANG Y Y, WANG J W, et al. Low-temperature and high-rate sodium metal batteries enabled by electrolyte chemistry[J]. Energy Storage Materials, 2022, 50: 47-54. DOI: 10.1016/j.ensm.2022.05.005. |
| 54 | FANG H Y, HUANG Y H, HU W, et al. Regulating ion-dipole interactions in weakly solvating electrolyte towards ultra-low temperature sodium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(15): 2400539. DOI: 10.1002/anie.202400539. |
| 55 | CHEN K A, SHEN X H, LUO L B, et al. Correlating the solvating power of solvents with the strength of ion-dipole interaction in electrolytes of lithium-ion batteries[J]. Angewandte Chemie, 2023, 135(47): e202312373. DOI: 10.1002/ange.202312373. |
| 56 | WANG X, YIN X P, FENG X C, et al. Rational design of Na0.67Ni0.2Co0.2Mn0.6O2 microsphere cathode material for stable and low temperature sodium ion storage[J]. Chemical Engineering Journal, 2022, 428: 130990. DOI: 10.1016/j.cej.2021.130990. |
| 57 | SHI Q H, QI R J, FENG X C, et al. Niobium-doped layered cathode material for high-power and low-temperature sodium-ion batteries[J]. Nature Communications, 2022, 13(1): 3205. DOI: 10.1038/s41467-022-30942-z. |
| 58 | ZHENG Y Q, SUN M Y, YU F D, et al. Utilizing weakly-solvated diglyme-based electrolyte to achieve a 10, 000-cycles durable Na3V2(PO4)2F3 cathode endured at -20 ℃[J]. Nano Energy, 2022, 102: 107693. DOI: 10.1016/j.nanoen.2022.107693. |
| 59 | ZHENG K X, XU S T, YAO Y, et al. Multi-component surface engineering of Na3V2(PO4)2O2F for low-temperature (-40 ℃) sodium-ion batteries[J]. Chemical Communications, 2022, 58(74): 10349-10352. DOI: 10.1039/d2cc03281c. |
| 60 | HUBBLE D, BROWN D E, ZHAO Y Z, et al. Liquid electrolyte development for low-temperature lithium-ion batteries[J]. Energy & Environmental Science, 2022, 15(2): 550-578. DOI: 10.1039/D1EE01789F. |
| 61 | ZHONG S E, YU Y S, YANG Y, et al. Molecular engineering on solvation structure of carbonate electrolyte toward durable sodium metal battery at -40 ℃[J]. Angewandte Chemie International Edition, 2023, 62(18): 2301169. DOI: 10.1002/anie.202301169. |
| 62 | SEOK J, HYUN J H, JIN A H, et al. Visualization of sodium metal anodes via Operando X-ray and optical microscopy: Controlling the morphological evolution of sodium metal plating[J]. ACS Applied Materials & Interfaces, 2022, 14(8): 10438-10446. DOI: 10.1021/acsami.1c24673. |
| 63 | CHENG H R, SUN Q J, LI L L, et al. Emerging era of electrolyte solvation structure and interfacial model in batteries[J]. ACS Energy Letters, 2022, 7(1): 490-513. DOI: 10.1021/acsenergylett.1c02425. |
| 64 | HAO H C, HUTTER T, BOYCE B L, et al. Review of multifunctional separators: Stabilizing the cathode and the anode for alkali (Li, Na, and K) metal-sulfur and selenium batteries[J]. Chemical Reviews, 2022, 122(9): 8053-8125. DOI: 10.1021/acs.chemrev.1c00838. |
| 65 | TAO L, RUSSELL J A, XIA D W, et al. Reversible switch in charge storage enabled by selective ion transport in solid electrolyte interphase[J]. Journal of the American Chemical Society, 2023, 145(30): 16538-16547. DOI: 10.1021/jacs.3c03429. |
| 66 | BOLLI C, GUÉGUEN A, MENDEZ M A, et al. Operando monitoring of F– formation in lithium ion batteries[J]. Chemistry of Materials, 2019, 31(4): 1258-1267. DOI: 10.1021/acs.chemmater.8b03810. |
| 67 | SONG X N, MENG T, DENG Y M, et al. The effects of the functional electrolyte additive on the cathode material Na0.76Ni0.3Fe0.4Mn0.3O2 for sodium-ion batteries[J]. Electrochimica Acta, 2018, 281: 370-377. DOI: 10.1016/j.electacta.2018.05.185. |
| 68 | WANG Q D, ZHAO C L, YAO Z P, et al. Entropy-driven liquid electrolytes for lithium batteries[J]. Advanced Materials, 2023, 35(17): e2210677. DOI: 10.1002/adma.202210677. |
| 69 | ZHANG W, XIA H R, ZHU Z Q, et al. Decimal solvent-based high-entropy electrolyte enabling the extended survival temperature of lithium-ion batteries to -130 ℃[J]. CCS Chemistry, 2021, 3(4): 1245-1255. DOI: 10.31635/ccschem.020.202000341. |
| 70 | TIAN Z N, ZOU Y G, LIU G, et al. Electrolyte solvation structure design for sodium ion batteries[J]. Advanced Science, 2022, 9(22): e2201207. DOI: 10.1002/advs.202201207. |
| 71 | YANG C Y, XIA J L, CUI C Y, et al. All-temperature zinc batteries with high-entropy aqueous electrolyte[J]. Nature Sustainability, 2023, 6: 325-335. DOI: 10.1038/s41893-022-01028-x. |
| 72 | YANG C, LIU X W, LIN Y, et al. Entropy-driven solvation toward low-temperature sodium-ion batteries with temperature-adaptive feature[J]. Advanced Materials, 2023, 35(28): 2301817. DOI: 10.1002/adma.202301817. |
| 73 | XIAO A W, LEE H J, CAPONE I, et al. Understanding the conversion mechanism and performance of monodisperse FeF2 nanocrystal cathodes[J]. Nature Materials, 2020, 19: 644-654. DOI: 10.1038/s41563-020-0621-z. |
| 74 | SUN H, ZHU G Z, ZHU Y M, et al. High-safety and high-energy-density lithium metal batteries in a novel ionic-liquid electrolyte[J]. Advanced Materials, 2020, 32(26): e2001741. DOI: 10.1002/adma.202001741. |
| 75 | KRUK D, JANCELEWICZ M, KLIMASZYK A, et al. Internal dynamics of ionic liquids over a broad temperature range—The role of the cation structure[J]. Materials, 2021, 15(1): 216. DOI: 10.3390/ma15010216. |
| 76 | SERRA MORENO J, MARESCA G, PANERO S, et al. Sodium-conducting ionic liquid-based electrolytes[J]. Electrochemistry Communications, 2014, 43: 1-4. DOI: 10.1016/j.elecom.2014.02.010. |
| 77 | LI P Y, HU N Q, WANG J Y, et al. Recent progress and perspective: Na ion batteries used at low temperatures[J]. Nanomaterials, 2022, 12(19): 3529. DOI: 10.3390/nano12193529. |
| 78 | DING C S, NOHIRA T, HAGIWARA R, et al. Electrochemical performance of hard carbon negative electrodes for ionic liquid-based sodium ion batteries over a wide temperature range[J]. Electrochimica Acta, 2015, 176: 344-349. DOI: 10.1016/j.electacta.2015.07.024. |
| 79 | BELLUSCI M, SIMONETTI E, DE FRANCESCO M, et al. Ionic liquid electrolytes for safer and more reliable sodium battery systems[J]. Applied Sciences, 2020, 10(18): 6323. DOI: 10.3390/app10186323. |
| 80 | DING C S, NOHIRA T, FUKUNAGA A, et al. Charge-discharge performance of an ionic liquid-based sodium secondary battery in a wide temperature range[J]. Electrochemistry, 2015, 83(2): 91-94. DOI: 10.5796/electrochemistry.83.91. |
| 81 | MATSUMOTO K, CHEN C Y, KIKO T, et al. Intermediate-temperature operation of sodium secondary batteries with high rate capability and cyclability using ionic liquid electrolyte[J]. ECS Transactions, 2016, 75(15): 139-145. DOI: 10.1149/07515.0139ecst. |
| 82 | GAO Y, YAN Z F, GRAY J L, et al. Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions[J]. Nature Materials, 2019, 18(4): 384-389. DOI: 10.1038/s41563-019-0305-8. |
| 83 | YANG Y X, ZHONG Y R, SHI Q W, et al. Electrocatalysis in lithium sulfur batteries under lean electrolyte conditions[J]. Angewandte Chemie International Edition, 2018, 57(47): 15549-15552. DOI: 10.1002/anie.201808311. |
| 84 | HU X F, MATIOS E, ZHANG Y W, et al. Deeply cycled sodium metal anodes at low temperature and in lean electrolyte conditions[J]. Angewandte Chemie International Edition, 2021, 60(11): 5978-5983. DOI: 10.1002/anie.202014241. |
| 85 | LIU Y, LU S W, WANG Z C, et al. Weakly polar ether-aided ionic liquid electrolyte enables high-performance sodium metal batteries over wide temperature range[J]. Advanced Functional Materials, 2024: 2312295. DOI: 10.1002/adfm.202312295. |
| [1] | Hong ZHOU, Zhulin XIN, Hao FU, Qiang ZHANG, Feng WEI. Analysis of the key materials employed in solid-state lithium batteries based on patent data mining [J]. Energy Storage Science and Technology, 2024, 13(7): 2386-2398. |
| [2] | Changhao LI, Shuping WANG, Xiankun YANG, Ziqi ZENG, Xinyue ZHOU, Jia XIE. Nonaqueous electrolyte in low-temperature lithium-ion battery [J]. Energy Storage Science and Technology, 2024, 13(7): 2286-2299. |
| [3] | Shirong TAN, Wenji YIN, Cuihong ZENG, Xiaoqiong LI, Shuo QI, Fangli JI, Sijiang HU, Hongqiang WANG, Qingyu LI. Role of high temperature quenching in structure and performance of Mn-based layered cathode materials for sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2399-2406. |
| [4] | Zongxun LI, Qiuqiu LYU, Haoyu ZHAO, Jianyu HE, Yang LIU, Zaihong SUN, Kaihua SUN, Tenglong ZHU. Research of GDC barrier layer applications by hydrothermal insitu growth in industrial-sized SOFC [J]. Energy Storage Science and Technology, 2024, 13(7): 2407-2413. |
| [5] | Sen JIANG, Long CHEN, Chuangchao SUN, Jinze WANG, Ruhong LI, Xiulin FAN. Low-temperature lithium battery electrolytes: Progress and perspectives [J]. Energy Storage Science and Technology, 2024, 13(7): 2270-2285. |
| [6] | Yang LU, Shuaishuai YAN, Xiao MA, Zhi LIU, Weili ZHANG, Kai LIU. Low-temperature electrolytes and their application in lithium batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2224-2242. |
| [7] | Shijie LIAO, Ying WEI, Yunhui HUANG, Renzong HU, Henghui XU. 1,3-Difluorobenzene diluent-stabilizing electrode interface for high-performance low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2124-2130. |
| [8] | Xiang LI, Dezhong LIU, Kai YUAN, Dapeng CHEN. Solid-state electrolyte for low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2327-2347. |
| [9] | Meilong WANG, Yurui XUE, Wenxi HU, Keyu DU, Ruitao SUN, Bin ZHANG, Ya YOU. Design and research of all-ether high-entropy electrolyte for low-temperature lithium iron phosphate batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2131-2140. |
| [10] | Wentao WANG, Yifan WEI, Kun HUANG, Guowei LV, Siyao ZHANG, Xinya TANG, Zeyan CHEN, Qingyuan LIN, Zhipeng MU, Kunhua WANG, Hua CAI, Jun CHEN. Testing standards and developmental advances for low-temperature Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2300-2307. |
| [11] | Zheng LI, Zhenzhong YANG, Qiong WANG, Renzong HU. Patent intelligence analysis of the research progress in low-temperature electrolytes for Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2317-2326. |
| [12] | Guangyu CHENG, Xinwei LIU, Shuo LIU, Haitao GU, Ke WANG. Controlling electrolyte solvent components to enhance cycle life of LCO/C low-temperature 18650 batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2171-2180. |
| [13] | Fei ZHAO, Yinghua CHEN, Zheng MA, Qian LI, Jun MING. Advances in low-temperature electrolytes for potassium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2308-2316. |
| [14] | Yuhao WANG, Zhiyong LI, Xin GUO. Applications and challenges of polymer-based electrolytes in low-temperature solid-state lithium batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2243-2258. |
| [15] | Junfeng HAO, Jing ZHU, Xiaoyu SHEN, Guanjun CEN, Ronghan QIAO, Xinxin ZHANG, Mengyu TIAN, Zhou JIN, Yuanjie ZHAN, Qiangfu SUN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Xuejie HUANG. A review of 100 selected recent studies on lithium batteries (April 1, 2024—May 31, 2024) [J]. Energy Storage Science and Technology, 2024, 13(7): 2361-2376. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
