Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (7): 2192-2205.doi: 10.19799/j.cnki.2095-4239.2024.0559
• Special Issue on Low Temperature Batteries • Previous Articles Next Articles
Zeheng LI1,2( ), Lei XU3, Yuxing YAO1, Chong YAN3, Ximin ZHAI4, Xuechun HAO4, Aibing CHEN5, Jiaqi HUANG3, Xiaofei BIE4, Huanli SUN4, Lizhen FAN6, Qiang ZHANG1,7,8(
), Lei XU3, Yuxing YAO1, Chong YAN3, Ximin ZHAI4, Xuechun HAO4, Aibing CHEN5, Jiaqi HUANG3, Xiaofei BIE4, Huanli SUN4, Lizhen FAN6, Qiang ZHANG1,7,8( )
)
Received:2024-06-21
Revised:2024-07-01
Online:2024-07-28
Published:2024-07-23
Contact:
Qiang ZHANG
E-mail:zehengli@zju.edu.cn;zhang-qiang@mails.tsinghua.edu.cn
CLC Number:
Zeheng LI, Lei XU, Yuxing YAO, Chong YAN, Ximin ZHAI, Xuechun HAO, Aibing CHEN, Jiaqi HUANG, Xiaofei BIE, Huanli SUN, Lizhen FAN, Qiang ZHANG. A review of electrolyte reducing lithium plating in low-temperature lithium-ion batteries[J]. Energy Storage Science and Technology, 2024, 13(7): 2192-2205.

Fig. 4
(a) A typical Nyquist plot of Li ion cell with well-separated semicircles and the corresponding equivalent circuit; (b) Temperature dependences of the Rb, RSEI, Rct, and Rct percentage of a full cell at 3.45 V[90]; (c) Cell voltage and electrode potential of NCM811||graphite pouch cells in 0.2C under -30 ℃; (d) Cycling performance of pouch cells at -30 ℃. Inset: the photo of a pouch cell[96]"
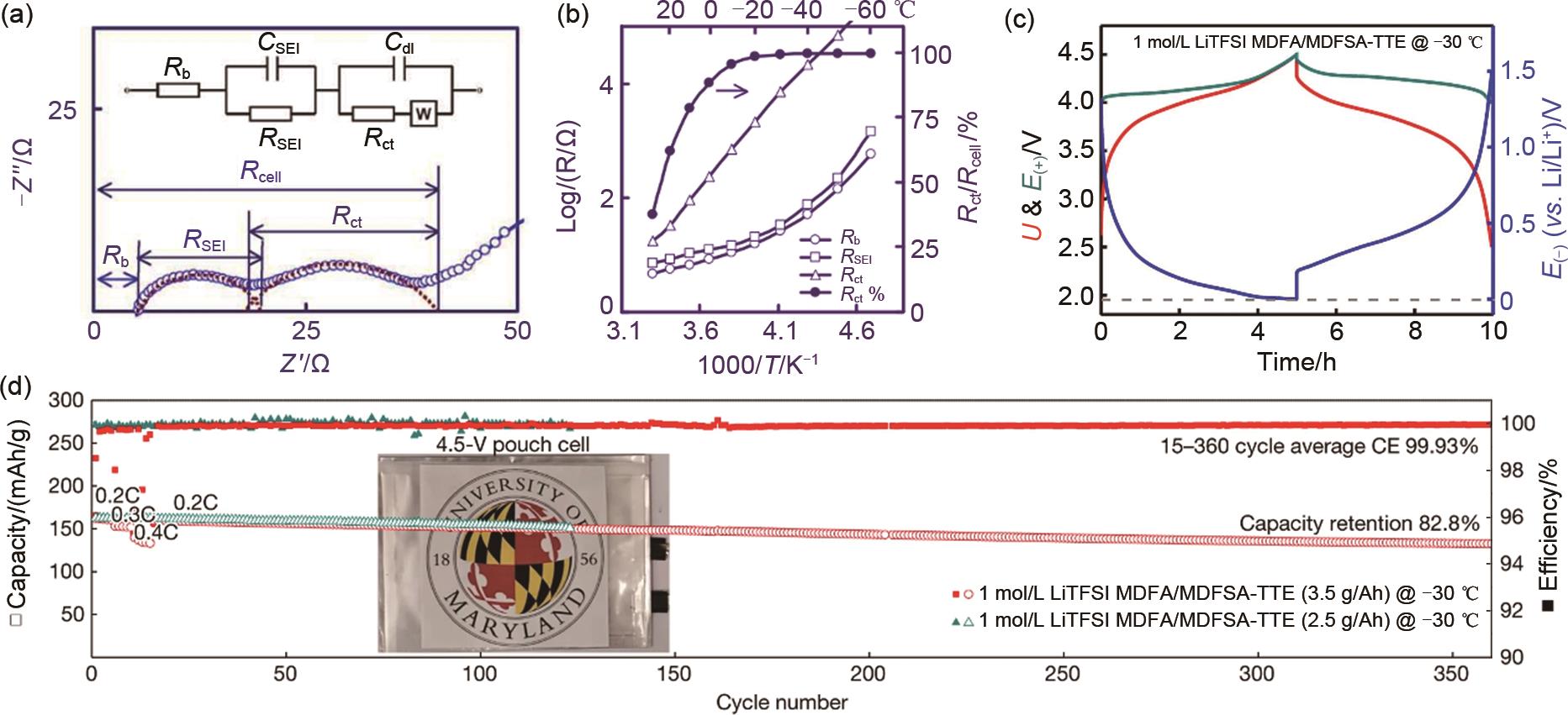

Fig. 5
(a) Comparison of LUMO and HOMO levels with the Fermi level of graphite and binding energy of lithium-solvents; (b) Schematic illustrations of lithiated graphite electrode using PC and DEGDME electrolytes[80]; (c) and (d) The SEM images of graphite electrode cycled at 0.5 and -40 ℃ in the graphite/Li half cell for 20 cycles; (e) Galvanostatic charge-discharge curves of graphite/Li half cell using the solvent co-intercalation electrolyte at 0.1 C and -60 ℃[99]"
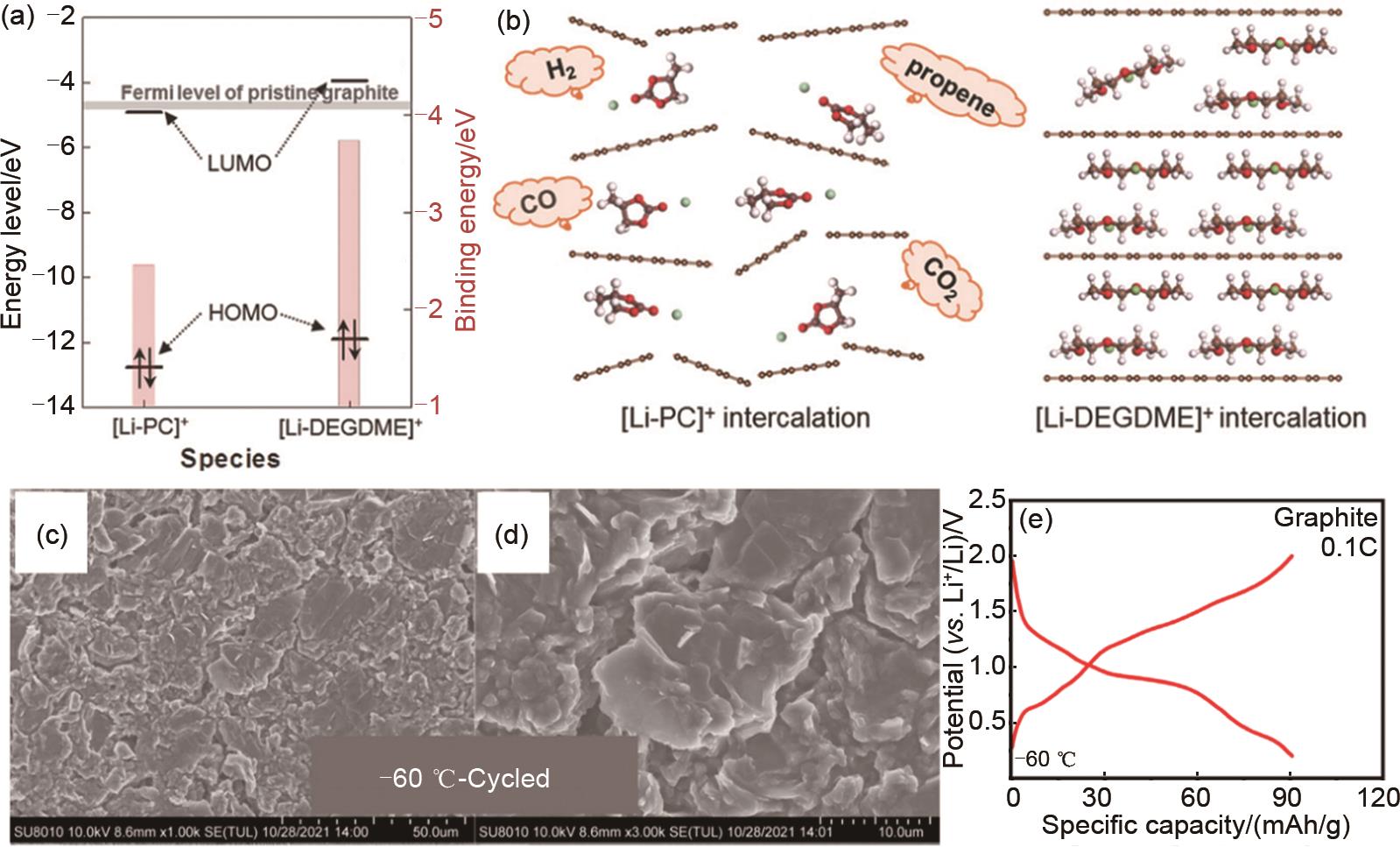
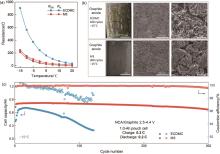
Fig. 6
(a) RSEI and Rct of the graphite electrodes obtained from EIS fitting at different temperatures using different electrolytes; (b) Surface morphology of graphite electrodes using different electrolytes at -15 ℃; (c) Cycling performances of NCA/graphite pouch full cells using different electrolytes at -15 ℃[28]"

| 1 | 李先锋, 张洪章, 郑琼, 等. 能源革命中的电化学储能技术[J]. 中国科学院院刊, 2019, 34(4): 443-449. DOI: 10.16418/j.issn.1000-3045.2019.04.009. |
| LI X F, ZHANG H Z, ZHENG Q, et al. Electrochemical energy storage technology in energy revolution[J]. Bulletin of Chinese Academy of Sciences, 2019, 34(4): 443-449. DOI: 10.16418/j.issn.1000-3045.2019.04.009. | |
| 2 | KABEYI M J B, OLANREWAJU O A. Sustainable energy transition for renewable and low carbon grid electricity generation and supply[J]. Frontiers in Energy Research, 2022, 9: 743114. DOI: 10.3389/fenrg.2021.743114. |
| 3 | ZOU C N, ZHAO Q, ZHANG G S, et al. Energy revolution: From a fossil energy era to a new energy era[J]. Natural Gas Industry B, 2016, 3(1): 1-11. DOI: 10.1016/j.ngib.2016.02.001. |
| 4 | SUN S, ZHAO C Z, YUAN H, et al. Multiscale understanding of high-energy cathodes in solid-state batteries: From atomic scale to macroscopic scale[J]. Materials Futures, 2022, 1(1): 012101. DOI: 10.1088/2752-5724/ac427c. |
| 5 | LIU H, CHENG X B, CHONG Y, et al. Advanced electrode processing of lithium ion batteries: A review of powder technology in battery fabrication[J]. Particuology, 2021, 57: 56-71. DOI: 10.1016/j.partic.2020.12.003. |
| 6 | ZHAO M, LI X Y, CHEN X, et al. Promoting the sulfur redox kinetics by mixed organodiselenides in high-energy-density lithium–sulfur batteries[J]. eScience, 2021, 1(1): 44-52. DOI: 10.1016/j.esci.2021.08.001. |
| 7 | FAN E S, LI L, WANG Z P, et al. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects[J]. Chemical Reviews, 2020, 120(14): 7020-7063. DOI: 10.1021/acs.chemrev.9b00535. |
| 8 | LI Z H, WAN Z W, ZENG X Q, et al. A robust network binder via localized linking by small molecules for high-areal-capacity silicon anodes in lithium-ion batteries[J]. Nano Energy, 2021, 79: 105430. DOI: 10.1016/j.nanoen.2020.105430. |
| 9 | LI M, LU J, CHEN Z W, et al. 30 years of lithium-ion batteries[J]. Advanced Materials, 2018: e1800561. DOI: 10.1002/adma.201800561. |
| 10 | CHOI J W, AURBACH D. Promise and reality of post-lithium-ion batteries with high energy densities[J]. Nature Reviews Materials, 2016, 1(4): 16013. DOI: 10.1038/natrevmats.2016.13. |
| 11 | YAN C, YAO Y X, CAI W L, et al. The influence of formation temperature on the solid electrolyte interphase of graphite in lithium ion batteries[J]. Journal of Energy Chemistry, 2020, 49: 335-338. DOI: 10.1016/j.jechem.2020.02.052. |
| 12 | CHENG X B, LIU H, YUAN H, et al. A perspective on sustainable energy materials for lithium batteries[J]. SusMat, 2021, 1(1): 38-50. DOI: 10.1002/sus2.4. |
| 13 | SHEN X, ZHANG X Q, DING F, et al. Advanced electrode materials in lithium batteries: Retrospect and prospect[J]. Energy Material Advances, 2021, 2021: 1205324. DOI: 10.34133/2021/1205324. |
| 14 | KULOVA T L, SKUNDIN A M. A critical review of electrode materials and electrolytes for Low-Temperature Lithium-Ion Batteries[J]. International Journal of Electrochemical Science, 2020, 15(9): 8638-8661. DOI: 10.20964/2020.09.50. |
| 15 | DONG X L, WANG Y G, XIA Y Y. Promoting rechargeable batteries operated at low temperature[J]. Accounts of Chemical Research, 2021, 54(20): 3883-3894. DOI: 10.1021/acs.accounts.1c00420. |
| 16 | CHEN M Z, ZHANG Y Y, XING G C, et al. Electrochemical energy storage devices working in extreme conditions[J]. Energy & Environmental Science, 2021, 14(6): 3323-3351. DOI: 10.1039/D1EE00271F. |
| 17 | HUBBLE D, BROWN D E, ZHAO Y Z, et al. Liquid electrolyte development for low-temperature lithium-ion batteries[J]. Energy & Environmental Science, 2022, 15(2): 550-578. DOI: 10.1039/D1EE01789F. |
| 18 | 梁君飞, 李艳梅, 袁浩, 等. 低温锂离子电池研究进展[J]. 北京航空航天大学学报, 2021, 47(11): 2155-2174. DOI: 10.13700/j.bh.1001-5965.2020.0587. |
| LIANG J F, LI Y M, YUAN H, et al. Research progress of low-temperature lithium-ion battery[J]. Journal of Beijing University of Aeronautics and Astronautics, 2021, 47(11): 2155-2174. DOI: 10.13700/j.bh.1001-5965.2020.0587. | |
| 19 | ZHANG N, DENG T, ZHANG S Q, et al. Critical review on low-temperature Li-ion/metal batteries[J]. Advanced Materials, 2022, 34(15): e2107899. DOI: 10.1002/adma.202107899. |
| 20 | WANG C N, XIE Y S, HUANG Y S, et al. Li3PO4-enriched SEI on graphite anode boosts Li+ de-solvation enabling fast-charging and low-temperature lithium-ion batteries[J]. Angewandte Chemie, 2024, 136(21): e202402301. DOI: 10.1002/ange.202402301. |
| 21 | CHEN J X, ZHANG J H, FAN X Z, et al. Solvating lithium and tethering aluminium using di-coordination-strength anions for low-temperature lithium metal batteries[J]. Energy & Environmental Science, 2024, 17(12): 4036-4043. DOI: 10.1039/D3EE03809B. |
| 22 | LIU H, CHENG X B, YAN C, et al. A perspective on energy chemistry of low-temperature lithium metal batteries[J]. iEnergy, 2022, 1(1): 72-81. DOI: 10.23919/IEN.2022.0003. |
| 23 | DUAN J Y, CHEN J X, WANG F F, et al. Ambiently fostering solid electrolyte interphase for low-temperature lithium metal batteries[J]. Journal of Energy Chemistry, 2023, 87: 473-478. DOI: 10.1016/j.jechem.2023.08.054. |
| 24 | HU D Z, CHEN G, TIAN J, et al. Unrevealing the effects of low temperature on cycling life of 21700-type cylindrical Li-ion batteries[J]. Journal of Energy Chemistry, 2021, 60: 104-110. DOI: 10.1016/j.jechem.2020.12.024. |
| 25 | XU L, XIAO Y, YANG Y, et al. Operando quantified lithium plating determination enabled by dynamic capacitance measurement in working Li-ion batteries[J]. Angewandte Chemie International Edition, 2022, 61(39): e202210365. DOI: 10.1002/anie.202210365. |
| 26 | LIU Q Q, DU C Y, SHEN B, et al. Understanding undesirable anode lithium plating issues in lithium-ion batteries[J]. RSC Advances, 2016, 6(91): 88683-88700. DOI: 10.1039/C6RA19482F. |
| 27 | LAFORGUE A, YUAN X Z, PLATT A, et al. Effects of fast charging at low temperature on a high energy Li-ion battery[J]. Journal of the Electrochemical Society, 2020, 167(14): 140521. DOI: 10.1149/1945-7111/abc4bc. |
| 28 | WALDMANN T, HOGG B I, WOHLFAHRT-MEHRENS M. Li plating as unwanted side reaction in commercial Li-ion cells–A review[J]. Journal of Power Sources, 2018, 384: 107-124. DOI: 10.1016/j.jpowsour.2018.02.063. |
| 29 | XU L, YANG Y, XIAO Y, et al. In-situ determination of onset lithium plating for safe Li-ion batteries[J]. Journal of Energy Chemistry, 2022, 67: 255-262. DOI: 10.1016/j.jechem.2021.10.016. |
| 30 | YAO Y X, YAO N, ZHOU X R, et al. Ethylene-carbonate-free electrolytes for rechargeable Li-ion pouch cells at sub-freezing temperatures[J]. Advanced Materials, 2022, 34(45): e2206448. DOI: 10.1002/adma.202206448. |
| 31 | LI Z H, YAO Y X, SUN S, et al. 40 years of low-temperature electrolytes for rechargeable lithium batteries[J]. Angewandte Chemie International Edition, 2023, 62(37): e202303888. DOI: 10.1002/anie.202303888. |
| 32 | NG B, COMAN P T, FAEGH E, et al. Low-temperature lithium plating/corrosion hazard in lithium-ion batteries: Electrode rippling, variable states of charge, and thermal and nonthermal runaway[J]. ACS Applied Energy Materials, 2020, 3(4): 3653-3664. DOI: 10.1021/acsaem.0c00130. |
| 33 | XU X Q, CHENG X B, JIANG F N, et al. Dendrite-accelerated thermal runaway mechanisms of lithium metal pouch batteries[J]. SusMat, 2022, 2(4): 435-444. DOI: 10.1002/sus2.74. |
| 34 | QIN Y D, ZUO P Y, CHEN X R, et al. An ultra-fast charging strategy for lithium-ion battery at low temperature without lithium plating[J]. Journal of Energy Chemistry, 2022, 72: 442-452. DOI: 10.1016/j.jechem.2022.05.010. |
| 35 | XU K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries[J]. Chemical Reviews, 2004, 104(10): 4303-4417. DOI: 10.1021/cr030203g. |
| 36 | ZHU G L, WEN K C, LV W Q, et al. Materials insights into low-temperature performances of lithium-ion batteries[J]. Journal of Power Sources, 2015, 300: 29-40. DOI: 10.1016/j.jpowsour.2015.09.056. |
| 37 | XU K. Electrolytes and interphases in Li-ion batteries and beyond[J]. Chemical Reviews, 2014, 114(23): 11503-11618. DOI: 10.1021/cr500003w. |
| 38 | 刘志浩, 杜童, 李瑞瑞, 等. 宽温域、高电压、安全无EC电解液研究进展[J]. 储能科学与技术, 2023, 12(8): 2504-2525. DOI: 10.19799/j.cnki.2095-4239.2023.0237. |
| LIU Z H, DU T, LI R R, et al. Developments of wide temperature range, high voltage and safe EC-free electrolytes[J]. Energy Storage Science and Technology, 2023, 12(8): 2504-2525. DOI: 10.19799/j.cnki.2095-4239.2023.0237. | |
| 39 | LIANG J Y, ZHANG Y Y, XIN S, et al. Mitigating swelling of the solid electrolyte interphase using an inorganic anion switch for low-temperature Lithium-ion batteries[J]. Angewandte Chemie International Edition, 2023, 62(16): e202300384. DOI: 10.1002/anie.202300384. |
| 40 | 封迈, 陈楠, 陈人杰. 锂离子电池低温电解液的研究进展[J]. 储能科学与技术, 2023, 12(3): 792-807. DOI: 10.19799/j.cnki.2095-4239.2022.0650. |
| FENG M, CHEN N, CHEN R J. Research progress of low-temperature electrolyte for lithium-ion battery[J]. Energy Storage Science and Technology, 2023, 12(3): 792-807. DOI: 10.19799/j.cnki.2095-4239.2022.0650. | |
| 41 | 胡华坤, 李新丽, 薛文东, 等. 基于CiteSpace的锂离子电池用低温电解液知识图谱分析[J]. 储能科学与技术, 2022, 11(1): 379-396. DOI: 10.19799/j.cnki.2095-4239.2021.0295. |
| HU H K, LI X L, XUE W D, et al. Knowledge map analysis of a low-temperature electrolyte for lithium-ion battery based on CiteSpace[J]. Energy Storage Science and Technology, 2022, 11(1): 379-396. DOI: 10.19799/j.cnki.2095-4239.2021.0295. | |
| 42 | LI Q, LIU G, CHENG H R, et al. Low-temperature electrolyte design for lithium-ion batteries: Prospect and challenges[J]. Chemistry, 2021, 27(64): 15842-15865. DOI: 10.1002/chem.202101407. |
| 43 | LU D, LI R H, RAHMAN M M, et al. Ligand-channel-enabled ultrafast Li-ion conduction[J]. Nature, 2024, 627(8002): 101-107. DOI: 10.1038/s41586-024-07045-4. |
| 44 | JIANG L L, YAN C, YAO Y X, et al. Inhibiting solvent co-intercalation in a graphite anode by a localized high-concentration electrolyte in fast-charging batteries[J]. Angewandte Chemie International Edition, 2021, 60(7): 3402-3406. DOI: 10.1002/anie.202009738. |
| 45 | ZOU Y G, MA Z, LIU G, et al. Non-flammable electrolyte enables high-voltage and wide-temperature lithium-ion batteries with fast charging[J]. Angewandte Chemie International Edition, 2023, 62(8): e202216189. DOI: 10.1002/anie.202216189. |
| 46 | LI Q Y, LU D P, ZHENG J M, et al. Li+-desolvation dictating lithium-ion battery's low-temperature performances[J]. ACS Applied Materials & Interfaces, 2017, 9(49): 42761-42768. DOI: 10.1021/acsami.7b13887. |
| 47 | PIAO N, GAO X N, YANG H C, et al. Challenges and development of lithium-ion batteries for low temperature environments[J]. eTransportation, 2022, 11: 100145. DOI: 10.1016/j.etran.2021.100145. |
| 48 | WILLIAMS M V, KUNZ H R, FENTON J M. Analysis of polarization curves to evaluate polarization sources in hydrogen/air PEM fuel cells[J]. Journal of the Electrochemical Society, 2005, 152(3): A635. DOI: 10.1149/1.1860034. |
| 49 | NIAN Q S, SUN T J, LIU S, et al. Issues and opportunities on low-temperature aqueous batteries[J]. Chemical Engineering Journal, 2021, 423: 130253. DOI: 10.1016/j.cej.2021.130253. |
| 50 | LI Z H, TANG W T, YANG Y J, et al. Engineering prelithiation of polyacrylic acid binder: A universal strategy to boost initial coulombic efficiency for high-areal-capacity Si-based anodes[J]. Advanced Functional Materials, 2022, 32(40): 2206615. DOI: 10.1002/adfm.202206615. |
| 51 | GUO Y B, CAI J L, LIAO Y L, et al. Insight into fast charging/discharging aging mechanism and degradation-safety analytics of 18650 lithium-ion batteries[J]. Journal of Energy Storage, 2023, 72: 108331. DOI: 10.1016/j.est.2023.108331. |
| 52 | JIN C B, YAO N, XIAO Y, et al. Taming solvent-solute interaction accelerates interfacial kinetics in low-temperature lithium-metal batteries[J]. Advanced Materials, 2023, 35(3): e2208340. DOI: 10.1002/adma.202208340. |
| 53 | MANN R F, AMPHLETT J C, PEPPLEY B A, et al. Application of Butler-Volmer equations in the modelling of activation polarization for PEM fuel cells[J]. Journal of Power Sources, 2006, 161(2): 775-781. DOI: 10.1016/j.jpowsour.2006.05.026. |
| 54 | MA T, NI Y X, WANG Q R, et al. Optimize lithium deposition at low temperature by weakly solvating power solvent[J]. Angewandte Chemie International Edition, 2022, 61(39): e202207927. DOI: 10.1002/anie.202207927. |
| 55 | HUANG Y Y, LI H Y, SHENG O W, et al. Recent progress on the low-temperature lithium metal batteries and electrolytes[J]. Advanced Sustainable Systems, 2023: 2300285. DOI: 10.1002/adsu.202300285. |
| 56 | HOLOUBEK J, LIU H D, WU Z H, et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature[J]. Nature Energy, 2021, 2021: 10.1038/s41560-10.1038/s41021-00783-z. DOI: 10.1038/s41560-021-00783-z. |
| 57 | GUPTA A, MANTHIRAM A. Designing advanced lithium-based batteries for low-temperature conditions[J]. Advanced Energy Materials, 2020, 10(38): 2001972. DOI: 10.1002/aenm.202001972. |
| 58 | KRACHKOVSKIY S A, BAZAK J D, WERHUN P, et al. Visualization of steady-state ionic concentration profiles formed in electrolytes during Li-ion battery operation and determination of mass-transport properties by in situ magnetic resonance imaging[J]. Journal of the American Chemical Society, 2016, 138(25): 7992-7999. DOI: 10.1021/jacs.6b04226. |
| 59 | LI X R, XU P C, TIAN Y, et al. Electrolyte modulators toward polarization-mitigated lithium-ion batteries for sustainable electric transportation[J]. Advanced Materials, 2022, 34(7): 2107787. DOI: 10.1002/adma.202107787. |
| 60 | CAI W L, YAN C, YAO Y X, et al. Rapid lithium diffusion in Order@Disorder pathways for fast-charging graphite anodes[J]. Small Structures, 2020, 1(1): 2070001. DOI: 10.1002/sstr.202070001. |
| 61 | CAI W L, YAO Y X, ZHU G L, et al. A review on energy chemistry of fast-charging anodes[J]. Chemical Society Reviews, 2020, 49(12): 3806-3833. DOI: 10.1039/c9cs00728h. |
| 62 | GALLAGHER K G, DEES D W, JANSEN A N, et al. A volume averaged approach to the numerical modeling of phase-transition intercalation electrodes presented for LixC6[J]. Journal of the Electrochemical Society, 2012, 159(12): A2029-A2037. DOI: 10.1149/2.015301jes. |
| 63 | CAI W L, YAN C, YAO Y X, et al. The boundary of lithium plating in graphite electrode for safe lithium-ion batteries[J]. Angewandte Chemie International Edition, 2021, 60(23): 13007-13012. DOI: 10.1002/anie.202102593. |
| 64 | VON LÜDERS C, ZINTH V, ERHARD S V, et al. Lithium plating in lithium-ion batteries investigated by voltage relaxation and in situ neutron diffraction[J]. Journal of Power Sources, 2017, 342: 17-23. DOI: 10.1016/j.jpowsour.2016.12.032. |
| 65 | HU J W, ZHONG S W, YAN T T. Using carbon black to facilitate fast charging in lithium-ion batteries[J]. Journal of Power Sources, 2021, 508: 230342. DOI: 10.1016/j.jpowsour.2021.230342. |
| 66 | YAO F, GÜNEŞ F, TA H Q, et al. Diffusion mechanism of lithium ion through basal plane of layered graphene[J]. Journal of the American Chemical Society, 2012, 134(20): 8646-8654. DOI: 10.1021/ja301586m. |
| 67 | JIN C B, LIU T F, SHENG O W, et al. Rejuvenating dead lithium supply in lithium metal anodes by iodine redox[J]. Nature Energy, 2021, 6: 378-387. DOI: 10.1038/s41560-021-00789-7. |
| 68 | ZHANG W B, SAYAVONG P, XIAO X, et al. Recovery of isolated lithium through discharged state calendar ageing[J]. Nature, 2024, 626(7998): 306-312. DOI: 10.1038/s41586-023-06992-8. |
| 69 | JIN C B, ZHANG X Q, SHENG O W, et al. Reclaiming inactive lithium with a triiodide/iodide redox couple for practical lithium metal batteries[J]. Angewandte Chemie International Edition, 2021, 60(42): 22990-22995. DOI: 10.1002/anie.202110589. |
| 70 | JIN C B, HUANG Y Y, LI L H, et al. A corrosion inhibiting layer to tackle the irreversible lithium loss in lithium metal batteries[J]. Nature Communications, 2023, 14(1): 8269. DOI: 10.1038/s41467-023-44161-7. |
| 71 | SHENG O W, WANG T Y, YANG T, et al. Passivating lithium metal anode by anti-corrosion concentrated ether electrolytes for longevity of batteries[J]. Nano Energy, 2024, 123: 109406. DOI: 10.1016/j.nanoen.2024.109406. |
| 72 | WANG J H, YAMADA Y, SODEYAMA K, et al. Superconcentrated electrolytes for a high-voltage lithium-ion battery[J]. Nature Communications, 2016, 7: 12032. DOI: 10.1038/ncomms12032. |
| 73 | SMART M C, RATNAKUMAR B V, SURAMPUDI S. Use of organic esters as cosolvents in electrolytes for lithium-ion batteries with improved low temperature performance[J]. Journal of the Electrochemical Society, 2002, 149(4): A361. DOI: 10.1149/1.1453407. |
| 74 | SMART M C, RATNAKUMAR B V, CHIN K B, et al. Lithium-ion electrolytes containing ester cosolvents for improved low temperature performance[J]. Journal of the Electrochemical Society, 2010, 157(12): A1361. DOI: 10.1149/1.3501236. |
| 75 | DONG X L, GUO Z W, GUO Z Y, et al. Organic batteries operated at -70℃[J]. Joule, 2018, 2(5): 902-913. DOI: 10.1016/j.joule.2018.01.017. |
| 76 | HALL D S, ELDESOKY A, LOGAN E R, et al. Exploring classes of co-solvents for fast-charging lithium-ion cells[J]. Journal of the Electrochemical Society, 2018, 165(10): A2365-A2373. DOI: 10.1149/2.1351810jes. |
| 77 | YANG Y, FANG Z, YIN Y, et al. Synergy of weakly-solvated electrolyte and optimized interphase enables graphite anode charge at low temperature[J]. Angewandte Chemie International Edition, 2022, 61(36): e202208345. DOI: 10.1002/anie.202208345. |
| 78 | YANG Y, LI P L, WANG N, et al. Fluorinated carboxylate ester-based electrolyte for lithium ion batteries operated at low temperature[J]. Chemical Communications, 2020, 56(67): 9640-9643. DOI: 10.1039/d0cc04049e. |
| 79 | DIVYA M L, LEE Y S, ARAVINDAN V. Solvent co-intercalation: An emerging mechanism in Li-, Na-, and K-ion capacitors[J]. ACS Energy Letters, 2021, 6(12): 4228-4244. DOI: 10.1021/acsenergylett.1c01801. |
| 80 | KIM H, LIM K, YOON G, et al. Exploiting lithium–ether co-intercalation in graphite for high-power lithium-ion batteries[J]. Advanced Energy Materials, 2017, 7(19): 1700418. DOI: 10.1002/aenm.201700418. |
| 81 | XU K. "Charge-transfer" process at graphite/electrolyte interface and the solvation sheath structure of Li+ in nonaqueous electrolytes[J]. Journal of the Electrochemical Society, 2007, 154(3): A162. DOI: 10.1149/1.2409866. |
| 82 | YAO N, CHEN X, FU Z H, et al. Applying classical, Ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries[J]. Chemical Reviews, 2022, 122(12): 10970-11021. DOI: 10.1021/acs.chemrev.1c00904. |
| 83 | ZHANG Y P, LI S Y, SHI J K, et al. Revealing the key role of non-solvating diluents for fast-charging and low temperature Li-ion batteries[J]. Journal of Energy Chemistry, 2024, 94: 171-180. DOI: 10.1016/j.jechem.2024.02.059. |
| 84 | SHI J K, XU C, LAI J W, et al. An amphiphilic molecule-regulated core-shell-solvation electrolyte for Li-metal batteries at ultra-low temperature[J]. Angewandte Chemie International Edition, 2023, 62(13): e202218151. DOI: 10.1002/anie.202218151. |
| 85 | LIAO B, LI H Y, XU M Q, et al. Designing low impedance interface films simultaneously on anode and cathode for high energy batteries[J]. Advanced Energy Materials, 2018, 8(22): 1800802. DOI: 10.1002/aenm.201800802. |
| 86 | PARK G, NAKAMURA H, LEE Y, et al. The important role of additives for improved lithium ion battery safety[J]. Journal of Power Sources, 2009, 189(1): 602-606. DOI: 10.1016/j.jpowsour.2008.09.088. |
| 87 | JURNG S, PARK S, YOON T, et al. Low-temperature performance improvement of graphite electrode by allyl sulfide additive and its film-forming mechanism[J]. Journal of the Electrochemical Society, 2016, 163(8): A1798-A1804. DOI: 10.1149/2.0051609jes. |
| 88 | XIE Z, HE J R, XIA Z Y, et al. Synergistic interphase modification with dual electrolyte additives to boost cycle stability of high nickel cathode for all-climate battery[J]. Journal of Energy Chemistry, 2023, 86: 197-207. DOI: 10.1016/j.jechem.2023.07.010. |
| 89 | XIA Z Y, ZHOU K, LIN X Y, et al. Rationally designing electrolyte additives for highly improving cyclability of LiNi0.5Mn1.5O4/Graphite cells[J]. Journal of Energy Chemistry, 2024, 91: 266-275. DOI: 10.1016/j.jechem.2023.11.045. |
| 90 | ZHANG S S, XU K, JOW T R. Electrochemical impedance study on the low temperature of Li-ion batteries[J]. Electrochimica Acta, 2004, 49(7): 1057-1061. DOI: 10.1016/j.electacta.2003.10.016. |
| 91 | LIU J P, YUAN B T, DONG L W, et al. Constructing low-solvation electrolytes for next-generation lithium-ion batteries[J]. Batteries & Supercaps, 2022, 5(10): 2200256. DOI: 10.1002/batt.202200256. |
| 92 | XIAO P T, YUN X R, CHEN Y F, et al. Insights into the solvation chemistry in liquid electrolytes for lithium-based rechargeable batteries[J]. Chemical Society Reviews, 2023, 52(15): 5255-5316. DOI: 10.1039/d3cs00151b. |
| 93 | LI T, ZHANG X Q, YAO N, et al. Stable anion-derived solid electrolyte interphase in lithium metal batteries[J]. Angewandte Chemie International Edition, 2021, 60(42): 22683-22687. DOI: 10.1002/anie.202107732. |
| 94 | LI Y, BAI F W, LI C Z, et al. Understanding the inorganic-rich feature of anion-derived solid electrolyte interphase[J]. Advanced Energy Materials, 2024, 14(21): 2304414. DOI: 10.1002/aenm.202304414. |
| 95 | YAO Y X, CHEN X, YAN C, et al. Regulating interfacial chemistry in lithium-ion batteries by a weakly solvating electrolyte[J]. Angewandte Chemie International Edition, 2021, 60(8): 4090-4097. DOI: 10.1002/anie.202011482. |
| 96 | XU J J, ZHANG J X, POLLARD T P, et al. Electrolyte design for Li-ion batteries under extreme operating conditions[J]. Nature, 2023, 614(7949): 694-700. DOI: 10.1038/s41586-022-05627-8. |
| 97 | QIN M S, ZENG Z Q, WU Q, et al. Microsolvating competition in Li+ solvation structure affording PC-based electrolyte with fast kinetics for lithium-ion batteries[J]. Advanced Functional Materials, 2024: 2406357. DOI: 10.1002/adfm.202406357. |
| 98 | QIN M S, ZENG Z Q, WU Q, et al. Dipole-dipole interactions for inhibiting solvent co-intercalation into a graphite anode to extend the horizon of electrolyte design[J]. Energy & Environmental Science, 2023, 16(2): 546-556. DOI: 10.1039/D2EE03626F. |
| 99 | YANG Y S, CHEN Y F, TAN L L, et al. Rechargeable LiNi0.65Co0.15Mn0.2O2||Graphite batteries operating at -60 ℃[J]. Angewandte Chemie International Edition, 2022, 61(42): 2209619. DOI: 10.1002/anie.202209619. |
| 100 | XIAO Y, XU R, YAN C, et al. A toolbox of reference electrodes for lithium batteries[J]. Advanced Functional Materials, 2022, 32(13): 2108449. DOI: 10.1002/adfm.202108449. |
| 101 | XIAO Y, XU L, BI C X, et al. Structural vulnerability control by encapsulation strategy toward durable lithium metal reference electrodes[J]. Advanced Energy Materials, 2024, 14(20): 2304502. DOI: 10.1002/aenm.202304502. |
| 102 | LU Y, ZHAO C Z, HUANG J Q, et al. The timescale identification decoupling complicated kinetic processes in lithium batteries[J]. Joule, 2022, 6(6): 1172-1198. DOI: 10.1016/j.joule.2022.05.005. |
| 103 | SUN S, ZHAO C Z, YUAN H, et al. Eliminating interfacial O-involving degradation in Li-rich Mn-based cathodes for all-solid-state lithium batteries[J]. Science Advances, 2022, 8(47): eadd5189. DOI: 10.1126/sciadv.add5189. |
| 104 | CAO X, JIA H, XU W, et al. Review—Localized high-concentration electrolytes for lithium batteries[J]. Journal of the Electrochemical Society, 2021, 168(1): 010522. DOI: 10.1149/1945-7111/abd60e. |
| 105 | QIN M S, LIU M C, ZENG Z Q, et al. Rejuvenating propylene carbonate-based electrolyte through nonsolvating interactions for wide-temperature Li-ions batteries[J]. Advanced Energy Materials, 2022, 12(48): 2201801. DOI: 10.1002/aenm.202201801. |
| 106 | QIN M S, ZENG Z Q, CHENG S J, et al. Two-dimensional electrolyte design: Broadening the horizons of functional electrolytes in lithium batteries[J]. Accounts of Chemical Research, 2024, 57(8): 1163-1173. DOI: 10.1021/acs.accounts.4c00022. |
| 107 | LI M, WANG C S, CHEN Z W, et al. New concepts in electrolytes[J]. Chemical Reviews, 2020, 120(14): 6783-6819. DOI: 10.1021/acs.chemrev.9b00531. |
| 108 | YAMADA Y, WANG J H, KO S, et al. Advances and issues in developing salt-concentrated battery electrolytes[J]. Nature Energy, 2019, 4: 269-280. DOI: 10.1038/s41560-019-0336-z. |
| 109 | REN Q M, LI Y W, SONG X S, et al. High voltage electrolytes for lithium batteries[J]. Progress in Chemistry, 2023, 35(7): 221132. |
| 110 | JIANG G X, LI F, WANG H P, et al. Perspective on high-concentration electrolytes for lithium metal batteries[J]. Small Structures, 2021, 2(5): 2000122. DOI: 10.1002/sstr.202000122. |
| 111 | XIE J D, PATRA J, CHANDRA RATH P, et al. Highly concentrated carbonate electrolyte for Li-ion batteries with lithium metal and graphite anodes[J]. Journal of Power Sources, 2020, 450: 227657. DOI: 10.1016/j.jpowsour.2019.227657. |
| 112 | ZHANG J G, XU W, XIAO J, et al. Lithium metal anodes with nonaqueous electrolytes[J]. Chemical Reviews, 2020, 120(24): 13312-13348. DOI: 10.1021/acs.chemrev.0c00275. |
| 113 | FLAMME B, RODRIGUEZ GARCIA G, WEIL M, et al. Guidelines to design organic electrolytes for lithium-ion batteries: Environmental impact, physicochemical and electrochemical properties[J]. Green Chemistry, 2017, 19(8): 1828-1849. DOI: 10.1039/C7GC00252A. |
| 114 | LI Z H, YAO N, YU L G, et al. Inhibiting gas generation to achieve ultralong-lifespan lithium-ion batteries at low temperatures[J]. Matter, 2023, 6(7): 2274-2292. DOI: 10.1016/j.matt.2023.04.012. |
| 115 | MÄRKER K, XU C, GREY C P. Operando NMR of NMC811/graphite lithium-ion batteries: Structure, dynamics, and lithium metal deposition[J]. Journal of the American Chemical Society, 2020, 142(41): 17447-17456. DOI: 10.1021/jacs.0c06727. |
| 116 | PAUL P P, THAMPY V, CAO C T, et al. Quantification of heterogeneous, irreversible lithium plating in extreme fast charging of lithium-ion batteries[J]. Energy & Environmental Science, 2021, 14(9): 4979-4988. DOI: 10.1039/D1EE01216A. |
| 117 | XU L, XIAO Y, YANG Y, et al. In situ Li-plating diagnosis for fast-charging Li-ion batteries enabled by relaxation-time detection[J]. Advanced Materials, 2023, 35(42): 2301881. DOI: 10.1002/adma.202301881. |
| 118 | LIU H, SUN X, CHENG X B, et al. Working principles of lithium metal anode in pouch cells[J]. Advanced Energy Materials, 2022, 12(47): 2202518. DOI: 10.1002/aenm.202202518. |
| 119 | LI Z H, WU Z Y, WU S X, et al. Designing advanced polymeric binders for high-performance rechargeable sodium batteries[J]. Advanced Functional Materials, 2024, 34(6): 2307261. DOI: 10.1002/adfm.202307261. |
| 120 | CHEN S R, NIU C J, LEE H, et al. Critical parameters for evaluating coin cells and pouch cells of rechargeable Li-metal batteries[J]. Joule, 2019, 3(4): 1094-1105. DOI: 10.1016/j.joule.2019.02.004. |
| 121 | CHAE S, CHOI S H, KIM N, et al. Integration of graphite and silicon anodes for the commercialization of high-energy lithium-ion batteries[J]. Angewandte Chemie International Edition, 2020, 59(1): 110-135. DOI: 10.1002/anie.201902085. |
| 122 | LI Z H, WU G, YANG Y J, et al. An ion-conductive grafted polymeric binder with practical loading for silicon anode with high interfacial stability in lithium-ion batteries[J]. Advanced Energy Materials, 2022, 12(29): 2201197. DOI: 10.1002/aenm.202201197. |
| 123 | XU Q, SUN J K, YIN Y X, et al. Facile synthesis of blocky SiOx/C with graphite-like structure for high-performance lithium-ion battery anodes[J]. Advanced Functional Materials, 2018, 28(8): 1705235. DOI: 10.1002/adfm.201705235. |
| 124 | HAN Z J, YABUUCHI N, SHIMOMURA K, et al. High-capacity Si-graphite composite electrodes with a self-formed porous structure by a partially neutralized polyacrylate for Li-ion batteries[J]. Energy & Environmental Science, 2012, 5(10): 9014-9020. DOI: 10.1039/C2EE22292B. |
| 125 | BERG C, MORASCH R, GRAF M, et al. Comparison of silicon and graphite anodes: Temperature-dependence of impedance characteristics and rate performance[J]. Journal of the Electrochemical Society, 2023, 170(3): 030534. DOI: 10.1149/1945-7111/acc09d. |
| [1] | Hong ZHOU, Zhulin XIN, Hao FU, Qiang ZHANG, Feng WEI. Analysis of the key materials employed in solid-state lithium batteries based on patent data mining [J]. Energy Storage Science and Technology, 2024, 13(7): 2386-2398. |
| [2] | Changhao LI, Shuping WANG, Xiankun YANG, Ziqi ZENG, Xinyue ZHOU, Jia XIE. Nonaqueous electrolyte in low-temperature lithium-ion battery [J]. Energy Storage Science and Technology, 2024, 13(7): 2286-2299. |
| [3] | Zongxun LI, Qiuqiu LYU, Haoyu ZHAO, Jianyu HE, Yang LIU, Zaihong SUN, Kaihua SUN, Tenglong ZHU. Research of GDC barrier layer applications by hydrothermal insitu growth in industrial-sized SOFC [J]. Energy Storage Science and Technology, 2024, 13(7): 2407-2413. |
| [4] | Sen JIANG, Long CHEN, Chuangchao SUN, Jinze WANG, Ruhong LI, Xiulin FAN. Low-temperature lithium battery electrolytes: Progress and perspectives [J]. Energy Storage Science and Technology, 2024, 13(7): 2270-2285. |
| [5] | Yang LU, Shuaishuai YAN, Xiao MA, Zhi LIU, Weili ZHANG, Kai LIU. Low-temperature electrolytes and their application in lithium batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2224-2242. |
| [6] | Shijie LIAO, Ying WEI, Yunhui HUANG, Renzong HU, Henghui XU. 1,3-Difluorobenzene diluent-stabilizing electrode interface for high-performance low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2124-2130. |
| [7] | Xiang LI, Dezhong LIU, Kai YUAN, Dapeng CHEN. Solid-state electrolyte for low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2327-2347. |
| [8] | Meilong WANG, Yurui XUE, Wenxi HU, Keyu DU, Ruitao SUN, Bin ZHANG, Ya YOU. Design and research of all-ether high-entropy electrolyte for low-temperature lithium iron phosphate batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2131-2140. |
| [9] | Jiaqi HUANG, Jieming XIONG, Enzhong TAN, Xinyu SUN, Liwei CHENG, Hua WANG. Revisiting the Na metal half-cell at low-temperature [J]. Energy Storage Science and Technology, 2024, 13(7): 2151-2160. |
| [10] | Xiongwen XU, Ying MO, Wang ZHOU, Huandong YAO, Juan HONG, Hua LEI, Jian TU, Jilei LIU. Effect of hard carbon kinetic properties on low-temperature performance of Na-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2141-2150. |
| [11] | Wentao WANG, Yifan WEI, Kun HUANG, Guowei LV, Siyao ZHANG, Xinya TANG, Zeyan CHEN, Qingyuan LIN, Zhipeng MU, Kunhua WANG, Hua CAI, Jun CHEN. Testing standards and developmental advances for low-temperature Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2300-2307. |
| [12] | Weiqi LIN, Qiaoyu LU, Yuhong CHEN, Linyuan QIU, Yurong JI, Lianyu GUAN, Xiang DING. Advances in cathode materials for low-temperature sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2348-2360. |
| [13] | Zheng LI, Zhenzhong YANG, Qiong WANG, Renzong HU. Patent intelligence analysis of the research progress in low-temperature electrolytes for Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2317-2326. |
| [14] | Guangyu CHENG, Xinwei LIU, Shuo LIU, Haitao GU, Ke WANG. Controlling electrolyte solvent components to enhance cycle life of LCO/C low-temperature 18650 batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2171-2180. |
| [15] | Fei ZHAO, Yinghua CHEN, Zheng MA, Qian LI, Jun MING. Advances in low-temperature electrolytes for potassium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2308-2316. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
