Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (7): 2308-2316.doi: 10.19799/j.cnki.2095-4239.2024.0426
• Special Issue on Low Temperature Batteries • Previous Articles Next Articles
Fei ZHAO1,2( ), Yinghua CHEN1,2, Zheng MA1, Qian LI1(
), Yinghua CHEN1,2, Zheng MA1, Qian LI1( ), Jun MING1,2(
), Jun MING1,2( )
)
Received:2024-05-13
Revised:2024-06-07
Online:2024-07-28
Published:2024-07-23
Contact:
Qian LI, Jun MING
E-mail:zhaojunhua@ciac.ac.cn;qianli@ciac.ac.cn;jun.ming@ciac.ac.cn
CLC Number:
Fei ZHAO, Yinghua CHEN, Zheng MA, Qian LI, Jun MING. Advances in low-temperature electrolytes for potassium-ion batteries[J]. Energy Storage Science and Technology, 2024, 13(7): 2308-2316.
Table 1
Composition and performance of low-temperature potassium-ion batteries"
| 电解质组分 | 温度 | 电池体系 | 电流密度/(mA/g) | 比容量/(mAh/g) | 循环 圈数 | 容量 保持率 | 功率密度/ (Wh/kg) |
|---|---|---|---|---|---|---|---|
| 1 mol/L KFSI in THF[ | 0 ℃ | 碳纳米纤维||石墨 | 50 | 200 | — | — | — |
| 0.91 mol/L KFSI in DEECl[ | -5 ℃ | 普鲁士蓝||石墨 | 20 | 65.5 | 80 | — | — |
| 1 mol/L KFSI in MTHF[ | -20 ℃ | 预钾化的3, 4, 9, 10-苝-四羧酸-二酐||石墨 | — | >100 | 100 | 94.38% | 197 |
| 0.4 mol/L KPF6 in DME+2 vol.% PDMS[ | -40 ℃ | 预钾化的3, 4, 9, 10-苝-四羧酸-二酐||无负极Cu | 26 | 82.8 | 50 | 82% | 152 |
| 1 mol/L KPF6 in DME+20 mmol/L LiNO3[ | -40 ℃ | 3, 4, 9, 10-苝-四羧酸-二酐||硬碳 | 65 | 89 | 100 | 79% | 157 |
| 4 mol/L KFSI in PC[ | 0 ℃ | 普鲁士白||石墨 | 200 | >60 | 1000 | 92.1% | — |
| 2 mol/L KCF3SO3 in H2O+HBCD[ | -20 ℃ | 六氰基铁酸铜||3, 4, 9, 10-苝四甲酰二亚胺-乙烯二胺共聚物 | — | >80 | 60 | 约100% | — |
| 10 mol/kg KCF3COO in H2O[ | -35 ℃ | K1.55Fe[Fe-(CN)6]0.95·1.03H2O||3,4,9,10-苝四羧酸二酰亚胺 | — | >90 | 1000 | 87.5% | 41.9 |
| KFSI-L[ | -40 ℃ | PHA@RP@BNC||PTCDA | 100 | >60 | 200 | 93.6% | — |
| 全氟磺酸树脂聚合物电解质+PC/EC混合溶液[ | -15 ℃ | 3, 4, 9, 10-苝-四羧酸-二酐||石墨 | 100 | 90.7 | 200 | 99.74% | — |

Fig. 2
The solvent strategy regulates the low-temperature performance of the electrolytes (a) The electron withdrawing effect of DEECl promotes the desolvation of K+, which achieves PB||graphite cells operating at -5 ℃[28]; (b) The weak solvation effect of MTHF promotes the desolvation of K+, which achieves KPTCDA||graphite cells operating at -20 ℃[29]"
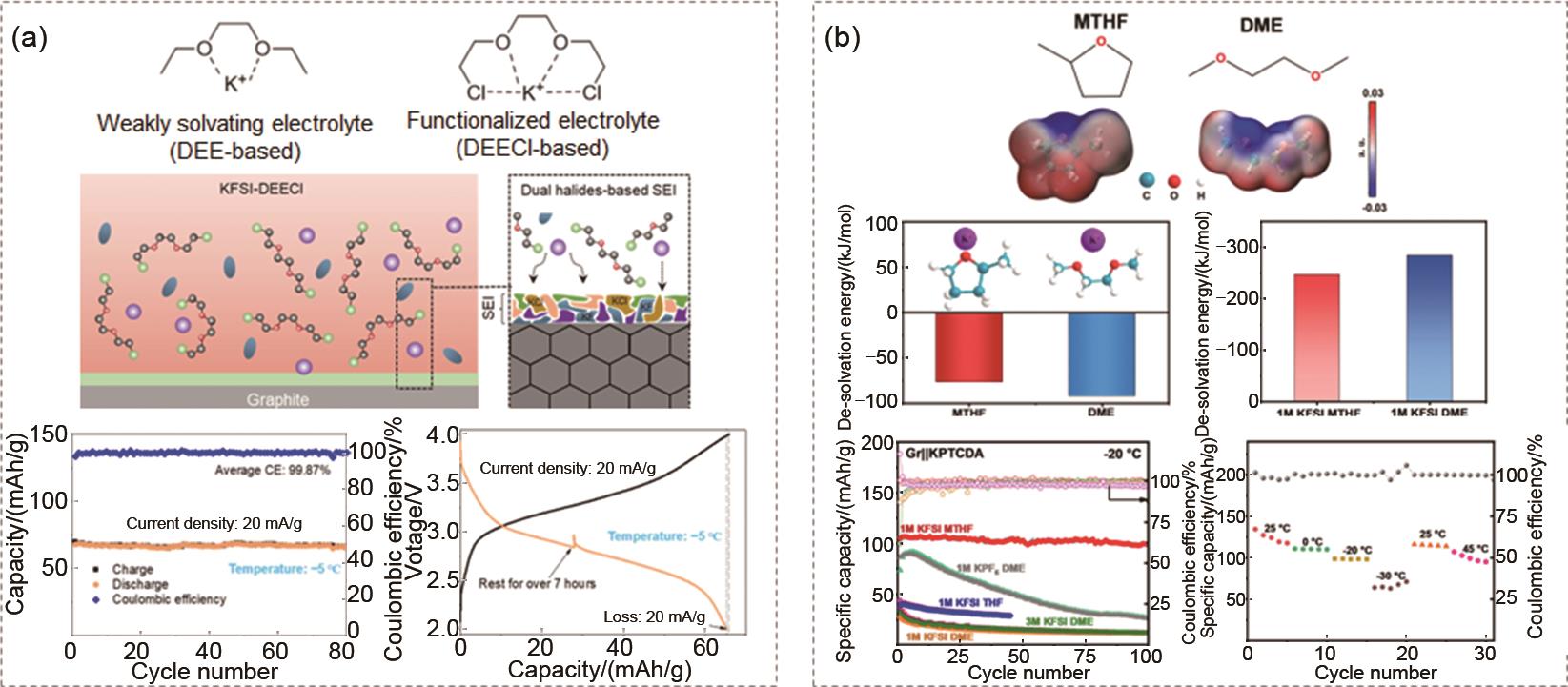

Fig. 5
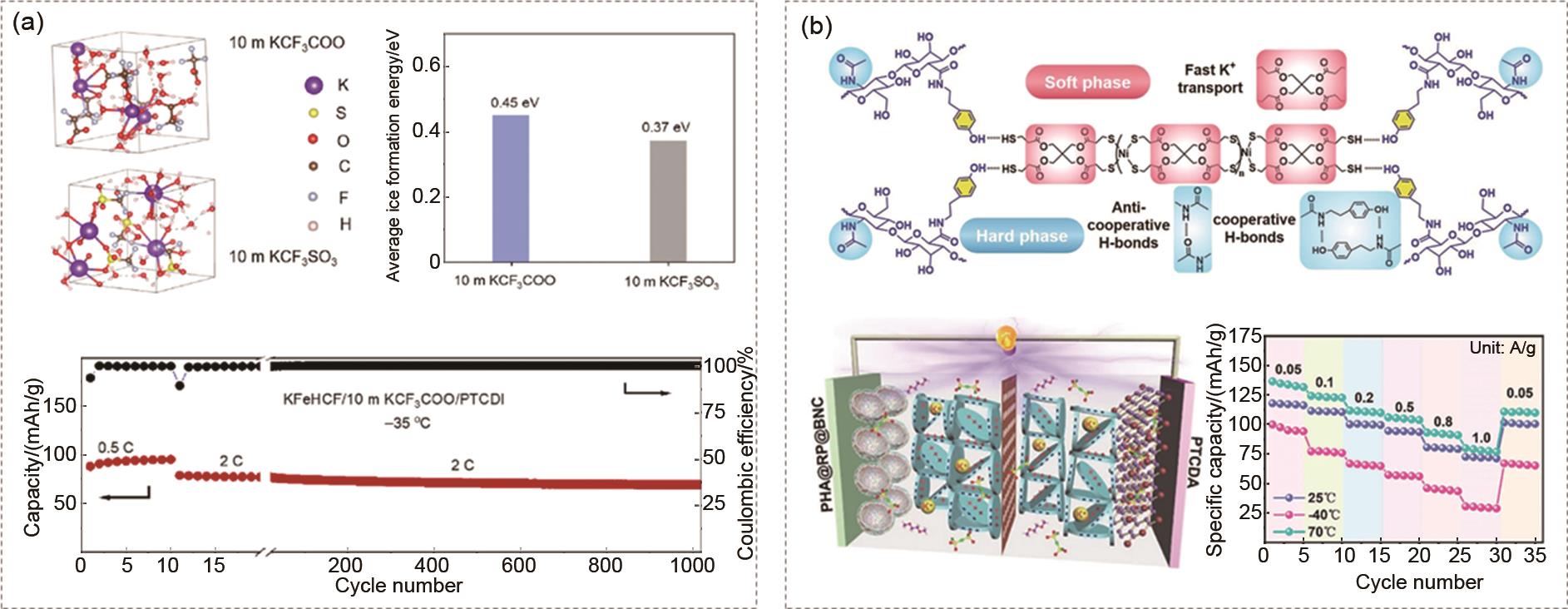
Design method of low temperature aqueous and solid-state electrolytes (a) Salt effect of KCF3COO regulates the low temperature aqueous electrolyte and make KFeHCF||PTCDI full cell achieves high energy density (41.9 Wh/kg) and long cycle life at -35 ℃ (1000 cycles, 2C, capacity retention of 87.5%)[43]; (b) In-situ polymerization of heptolite packing, PETMP-Ni and modified B, N, and red phosphorus (RP)-coated carbon spheres (BNC) to form a gel electrolyte and make the capacity retention rate of PTCDA||PHA@RP@BNC full cell at -40 ℃ that works 200 cycles is 93.6%[14]"
| 1 | TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414(6861): 359-367. DOI: 10.1038/35104644. |
| 2 | XU Y F, DING T J, SUN D M, et al. Recent advances in electrolytes for potassium-ion batteries[J]. Advanced Functional Materials, 2023, 33(6): 2211290. DOI: 10.1002/adfm.202211290. |
| 3 | WANG H W, ZHAI D Y, KANG F Y. Solid electrolyte interphase (SEI) in potassium ion batteries[J]. Energy & Environmental Science, 2020, 13(12): 4583-4608. DOI: 10.1039/d0ee01638a. |
| 4 | HOSAKA T, KUBOTA K, HAMEED A S, et al. Research development on K-ion batteries[J]. Chemical Reviews, 2020, 120(14): 6358-6466. DOI: 10.1021/acs.chemrev.9b00463. |
| 5 | KOMABA S, ITABASHI T, KIMURA T, et al. Opposite influences of K+ versus Na+ ions as electrolyte additives on graphite electrode performance[J]. Journal of Power Sources, 2005, 146(1/2): 166-170. DOI: 10.1016/j.jpowsour.2005.03.121. |
| 6 | JIAN Z L, LUO W, JI X L. Carbon electrodes for K-ion batteries[J]. Journal of the American Chemical Society, 2015, 137(36): 11566-11569. DOI: 10.1021/jacs.5b06809. |
| 7 | LUO W, WAN J Y, OZDEMIR B, et al. Potassium ion batteries with graphitic materials[J]. Nano Letters, 2015, 15(11): 7671-7677. DOI: 10.1021/acs.nanolett.5b03667. |
| 8 | ZHAO J, ZOU X X, ZHU Y J, et al. Electrochemical intercalation of potassium into graphite[J]. Advanced Functional Materials, 2016, 26(44): 8103-8110. DOI: 10.1002/adfm.201602248. |
| 9 | FAN L, MA R F, ZHANG Q F, et al. Graphite anode for a potassium-ion battery with unprecedented performance[J]. Angewandte Chemie International Edition, 2019, 58(31): 10500-10505. DOI: 10.1002/anie.201904258. |
| 10 | HARPER G, SOMMERVILLE R, KENDRICK E, et al. Recycling lithium-ion batteries from electric vehicles[J]. Nature, 2019, 575: 75-86. DOI: 10.1038/s41586-019-1682-5. |
| 11 | LÜCK J, LATZ A. A double-layer model for describing the effect of solvation and adsorption of ions on electrode surfaces in batteries[J]. ECS Meeting Abstracts, 2015, (2): 193. DOI: 10.1149/ma2015-02/2/193. |
| 12 | LIU J L, YIN T T, TIAN B B, et al. Unraveling the potassium storage mechanism in graphite foam[J]. Advanced Energy Materials, 2019, 9(22): 1900579. DOI: 10.1002/aenm.201900579. |
| 13 | WU X, CHEN Y L, XING Z, et al. Advanced carbon-based anodes for potassium-ion batteries[J]. Advanced Energy Materials, 2019, 9(21): 1900343. DOI: 10.1002/aenm.201900343. |
| 14 | QIN G H, ZHANG Y B, QI Z G, et al. Dynamically reversible gelation of electrolyte for efficient wide-temperature adaptable energy storage[J]. Advanced Functional Materials, 2024: 2316813. DOI: 10.1002/adfm.202316813. |
| 15 | DU G Y, TAO M L, LIU D Y, et al. Low-operating temperature quasi-solid-state potassium-ion battery based on commercial materials[J]. Journal of Colloid and Interface Science, 2021, 582: 932-939. DOI: 10.1016/j.jcis.2020.08.069. |
| 16 | LI Q, LIU G, CHENG H R, et al. Low-temperature electrolyte design for lithium-ion batteries: Prospect and challenges[J]. Chemistry, 2021, 27(64): 15842-15865. DOI: 10.1002/chem.202101407. |
| 17 | MING J, CAO Z, LI Q, et al. Molecular-scale interfacial model for predicting electrode performance in rechargeable batteries[J]. ACS Energy Letters, 2019, 4(7): 1584-1593. DOI: 10.1021/acsenergylett.9b00822. |
| 18 | QIN L, XIAO N, ZHENG J F, et al. Localized high-concentration electrolytes boost potassium storage in high-loading graphite[J]. Advanced Energy Materials, 2019, 9(44): 1902618. DOI: 10.1002/aenm.201902618. |
| 19 | LI L, LIU L J, HU Z, et al. Understanding high-rate K+-solvent co-intercalation in natural graphite for potassium-ion batteries[J]. Angewandte Chemie, 2020, 132(31): 13017-13024. DOI: 10.1002/ange.202001966. |
| 20 | 程浩然, 马征, 郭营军, 等. 影响电池性能的因素: 金属离子溶剂化结构衍生的界面行为还是固体电解质界面膜?[J]. 电化学, 2022, 28(11): 51-73. DOI: 10.13208/j.electrochem.2219012. |
| CHENG H R, MA Z, GUO Y J, et al. Which factor dominates battery performance: Metal ion solvation structure-derived interfacial behavior or solid electrolyte interphase layer?[J]. Journal of Electrochemistry, 2022, 28(11): 51-73. DOI: 10.13208/j.electrochem.2219012. | |
| 21 | LIU S L, MAO J F, ZHANG L, et al. Manipulating the solvation structure of nonflammable electrolyte and interface to enable unprecedented stability of graphite anodes beyond 2 years for safe potassium-ion batteries[J]. Advanced Materials, 2021, 33(1): e2006313. DOI: 10.1002/adma.202006313. |
| 22 | DIVYA M L, LEE Y S, ARAVINDAN V. Solvent co-intercalation: An emerging mechanism in Li-, Na-, and K-ion capacitors[J]. ACS Energy Letters, 2021, 6(12): 4228-4244. DOI: 10.1021/acsenergylett.1c01801. |
| 23 | CHENG H R, SUN Q J, LI L L, et al. Emerging era of electrolyte solvation structure and interfacial model in batteries[J]. ACS Energy Letters, 2022, 7(1): 490-513. DOI: 10.1021/acsenergylett.1c02425. |
| 24 | WANG Y Q, CAO Z, MA Z, et al. Weak solvent-solvent interaction enables high stability of battery electrolyte[J]. ACS Energy Letters, 2023, 8(3): 1477-1484. DOI: 10.1021/acsenergylett.3c00052. |
| 25 | CAI T, WANG Y Q, ZHAO F, et al. Graphic, quantitation, visualization, standardization, digitization, and intelligence of electrolyte and electrolyte-electrode interface[J]. Advanced Energy Materials, 2024: 2400569. DOI: 10.1002/aenm.202400569. |
| 26 | CHENG H R, MA Z, LI Q, et al. Design of new chemicals for advanced electrolytes[J]. Science China Chemistry, 2024, 67(5): 1378-1380. DOI: 10.1007/s11426-023-1716-7. |
| 27 | YU Z L, LIU Q, CHEN C S, et al. Regulating the interfacial chemistry enables fast-kinetics hard carbon anodes for potassium ion batteries[J]. Journal of Power Sources, 2023, 557: 232592. DOI: 10.1016/j.jpowsour.2022.232592. |
| 28 | HU Y Y, FU H W, GENG Y H, et al. Chloro-functionalized ether-based electrolyte for high-voltage and stable potassium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(23): 2403269. DOI: 10.1002/anie.202403269. |
| 29 | CHENG L W, LAN H, GAO Y, et al. Realizing low-temperature graphite-based rechargeable potassium-ion full battery[J]. Angewandte Chemie International Edition, 2024, 63(7): 2315624. DOI: 10.1002/anie.202315624. |
| 30 | LIU G, CAO Z, ZHOU L, et al. Additives engineered nonflammable electrolyte for safer potassium ion batteries[J]. Advanced Functional Materials, 2020, 30(43): 2001934. DOI: 10.1002/adfm.202001934. |
| 31 | NI L, XU G J, LI C C, et al. Electrolyte formulation strategies for potassium-based batteries[J]. Exploration, 2022, 2(2): 20210239. DOI: 10.1002/EXP.20210239. |
| 32 | LUO K, WANG Z X, MO Y, et al. Potassium selenocyanate (KSeCN) additive enabled stable cathode electrolyte interphase and iron dissolution inhibition toward long-cycling potassium-ion batteries[J]. Advanced Functional Materials, 2024, 34(13): 2311364. DOI: 10.1002/adfm.202311364. |
| 33 | TANG M Y, DONG S, WANG J W, et al. Low-temperature anode-free potassium metal batteries[J]. Nature Communications, 2023, 14(1): 6006. DOI: 10.1038/s41467-023-41778-6. |
| 34 | CHEN J C, AN D, WANG S C, et al. Rechargeable potassium-ion full cells operating at -40 ℃[J]. Angewandte Chemie International Edition, 2023, 62(33): e202307122. DOI: 10.1002/anie.202307122. |
| 35 | KITANI A, FUKUTA T, KODAMA Y, et al. Suppression of decomposition of propylene carbonate at graphite electrodes by the addition of nonionic surfactants[J]. Electrochemistry, 2003, 71(12): 1076-1077. DOI: 10.5796/electrochemistry.71.1076. |
| 36 | ALVARADO J, SCHROEDER M A, ZHANG M H, et al. A carbonate-free, sulfone-based electrolyte for high-voltage Li-ion batteries[J]. Materials Today, 2018, 21(4): 341-353. DOI: 10.1016/j.mattod.2018.02.005. |
| 37 | ZHANG J, CAO Z, ZHOU L, et al. Model-based design of graphite-compatible electrolytes in potassium-ion batteries[J]. ACS Energy Letters, 2020, 5(8): 2651-2661. DOI: 10.1021/acsenergylett.0c01401. |
| 38 | ZHANG J, CAO Z, ZHOU L, et al. Model-based design of stable electrolytes for potassium ion batteries[J]. ACS Energy Letters, 2020, 5(10): 3124-3131. DOI: 10.1021/acsenergylett.0c01634. |
| 39 | WANG Z X, LUO K, WU J F, et al. Rejuvenating propylene carbonate-based electrolytes by regulating the coordinated structure toward all-climate potassium-ion batteries[J]. Energy & Environmental Science, 2024, 17(1): 274-283. DOI: 10.1039/D3EE03340F. |
| 40 | LIN R, KE C M, CHEN J E, et al. Asymmetric donor-acceptor molecule-regulated core-shell-solvation electrolyte for high-voltage aqueous batteries[J]. Joule, 2022, 6(2): 399-417. DOI: 10.1016/j.joule.2022.01.002. |
| 41 | NIAN Q S, WANG J Y, LIU S, et al. Aqueous batteries operated at -50 ℃[J]. Angewandte Chemie International Edition, 2019, 58(47): 16994-16999. DOI: 10.1002/anie.201908913. |
| 42 | WEI J, ZHANG P B, LIU Y Z, et al. Wide-voltage-window amphiphilic supramolecule excluded-volume electrolytes for ultra-durable full-cell aqueous potassium-Ion batteries[J]. Chemical Engineering Journal, 2023, 459: 141623. DOI: 10.1016/j.cej.2023.141623. |
| 43 | JIANG L W, LU Y C. Building a long-lifespan aqueous K-ion battery operating at –35 ℃[J]. ACS Energy Letters, 2024, 9(3): 985-991. DOI: 10.1021/acsenergylett.4c00098. |
| 44 | MANTHIRAM A, YU X W, WANG S F. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2(4): 16103. DOI: 10.1038/natrevmats.2016.103. |
| 45 | XU L, TANG S, CHENG Y, et al. Interfaces in solid-state lithium batteries[J]. Joule, 2018, 2(10): 1991-2015. DOI: 10.1016/j.joule.2018.07.009. |
| 46 | XU S J, SUN Z H, SUN C G, et al. Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature[J]. Advanced Functional Materials, 2020, 30(51): 2007172. DOI: 10.1002/adfm.202007172. |
| 47 | XU S J, XU R G, YU T, et al. Decoupling of ion pairing and ion conduction in ultrahigh-concentration electrolytes enables wide-temperature solid-state batteries[J]. Energy & Environmental Science, 2022, 15(8): 3379-3387. DOI: 10.1039/D2EE01053D. |
| 48 | ZHAO T, ZHENG X Y, WANG D H, et al. A quasi-solid-state polyether electrolyte for low-temperature sodium metal batteries[J]. Advanced Functional Materials, 2023, 33(48): 2304928. DOI: 10.1002/adfm.202304928. |
| [1] | Meilong WANG, Yurui XUE, Wenxi HU, Keyu DU, Ruitao SUN, Bin ZHANG, Ya YOU. Design and research of all-ether high-entropy electrolyte for low-temperature lithium iron phosphate batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2131-2140. |
| [2] | Wentao WANG, Yifan WEI, Kun HUANG, Guowei LV, Siyao ZHANG, Xinya TANG, Zeyan CHEN, Qingyuan LIN, Zhipeng MU, Kunhua WANG, Hua CAI, Jun CHEN. Testing standards and developmental advances for low-temperature Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2300-2307. |
| [3] | Mingming SUN. Patent analysis of organic-inorganic composite solid-state electrolytes for lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(3): 1096-1105. |
| [4] | Feng LI, Xiaobin CHENG, Jinda LUO, Hongbin YAO. Metal chloride solid-state electrolytes and all-solid-state batteries: State-of-the-art developments and perspectives [J]. Energy Storage Science and Technology, 2024, 13(1): 193-211. |
| [5] | Huan LIU, Na PENG, Qingwen GAO, Wenpeng LI, Zhirong YANG, Jingtao WANG. Crown ether-doped polymer solid electrolyte for high-performance all-solid-state lithium batteries [J]. Energy Storage Science and Technology, 2023, 12(8): 2401-2411. |
| [6] | Lei LEI, Peng GAO, Nana FENG, Kunpeng CAI, Hai ZHANG, Yang ZHANG. The influences of multifactors in the synthesis progress on the characteristics of lithium lanthanum zirconate solid electrolytes [J]. Energy Storage Science and Technology, 2023, 12(5): 1625-1635. |
| [7] | Xuanchen WANG, Da WANG, Zhaomeng LIU, Xuanwen GAO, Wenbin LUO. Research progress and prospect of potassium ion battery electrolyte [J]. Energy Storage Science and Technology, 2023, 12(5): 1409-1426. |
| [8] | Ziwei YUAN, Chuyuan LIN, Ziyan YUAN, Xiaoli SUN, Qingrong QIAN, Qinghua CHEN, Lingxing ZENG. The research process on low temperature performance of zinc ion batteries [J]. Energy Storage Science and Technology, 2023, 12(1): 278-298. |
| [9] | LI Yitao, SHEN Kaier, PANG Quanquan. Advance in organics enhanced sulfide-based solid-state batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1902-1918. |
| [10] | Qingwen GAO, Zhihao YANG, Wenpeng LI, Wenjia WU, Jingtao WANG. Preparation and performance of Co2+-doped CeO2-based laminar composite solid-state electrolyte [J]. Energy Storage Science and Technology, 2022, 11(12): 3776-3786. |
| [11] | Xinxin ZHU, Wei JIANG, Zhengwei WAN, Shu ZHAO, Zeheng LI, Liguang WANG, Wenbin NI, Min LING, Chengdu LIANG. Research progress in electrolyte and interfacial issues of solid lithium sulfur batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 848-862. |
| [12] | Shangsen CHI, Yidong JIANG, Qingrong WANG, Ziwei YE, Kai YU, Jun MA, Jun JIN, Jun WANG, Chaoyang WANG, Zhaoyin WEN, Yonghong DENG. The liquid electrolyte modified interface between garnet-type solid-state electrolyte and lithium anode [J]. Energy Storage Science and Technology, 2021, 10(3): 914-924. |
| [13] | Wenlin YAN, Fan WU, Hong LI, Liquan CHEN. Application of Si-based anodes in sulfide solid-state batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 821-835. |
| [14] | Yongsheng GAO, Guanghai CHEN, Xinran WANG, Ying BAI, Chuan WU. Safety of electrolytes for sodium-ion batteries: Strategies and progress [J]. Energy Storage Science and Technology, 2020, 9(5): 1309-1317. |
| [15] | ZHOU Hong, WEI Feng, WU Yongqing. Research on the development of inorganic solid-state electrolyte for lithium battery based on patent analysis [J]. Energy Storage Science and Technology, 2020, 9(3): 1001-1007. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
