Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (8): 3004-3018.doi: 10.19799/j.cnki.2095-4239.2025.0519
• Special Issue on Short Term High-Frequency High-Power Energy Storage • Previous Articles
Fuxu XING1( ), Qi QIN1, Longkang WANG1, Yubing LI2, Shuaikai XU2, Tangming MO1(
), Qi QIN1, Longkang WANG1, Yubing LI2, Shuaikai XU2, Tangming MO1( )
)
Received:2025-06-03
Revised:2025-06-25
Online:2025-08-28
Published:2025-08-18
Contact:
Tangming MO
E-mail:xingfuxu410@163.com;motangming@gxu.edu.cn
CLC Number:
Fuxu XING, Qi QIN, Longkang WANG, Yubing LI, Shuaikai XU, Tangming MO. Recent advances in theoretical and computational simulations of pseudocapacitors[J]. Energy Storage Science and Technology, 2025, 14(8): 3004-3018.

Fig. 3
(a) Schematic illustrations and corresponding electrochemical responses of intrinsic and extrinsic pseudocapacitors; (b) Extrinsic pseudocapacitance, where high specific capacitance and pseudocapacitive behavior are only observed at the material surface; (c) Intrinsic pseudocapacitance of Nb2O5, where pseudocapacitive behavior is maintained even in thick films due to rapid Li+ transport within the bulk material[23]"
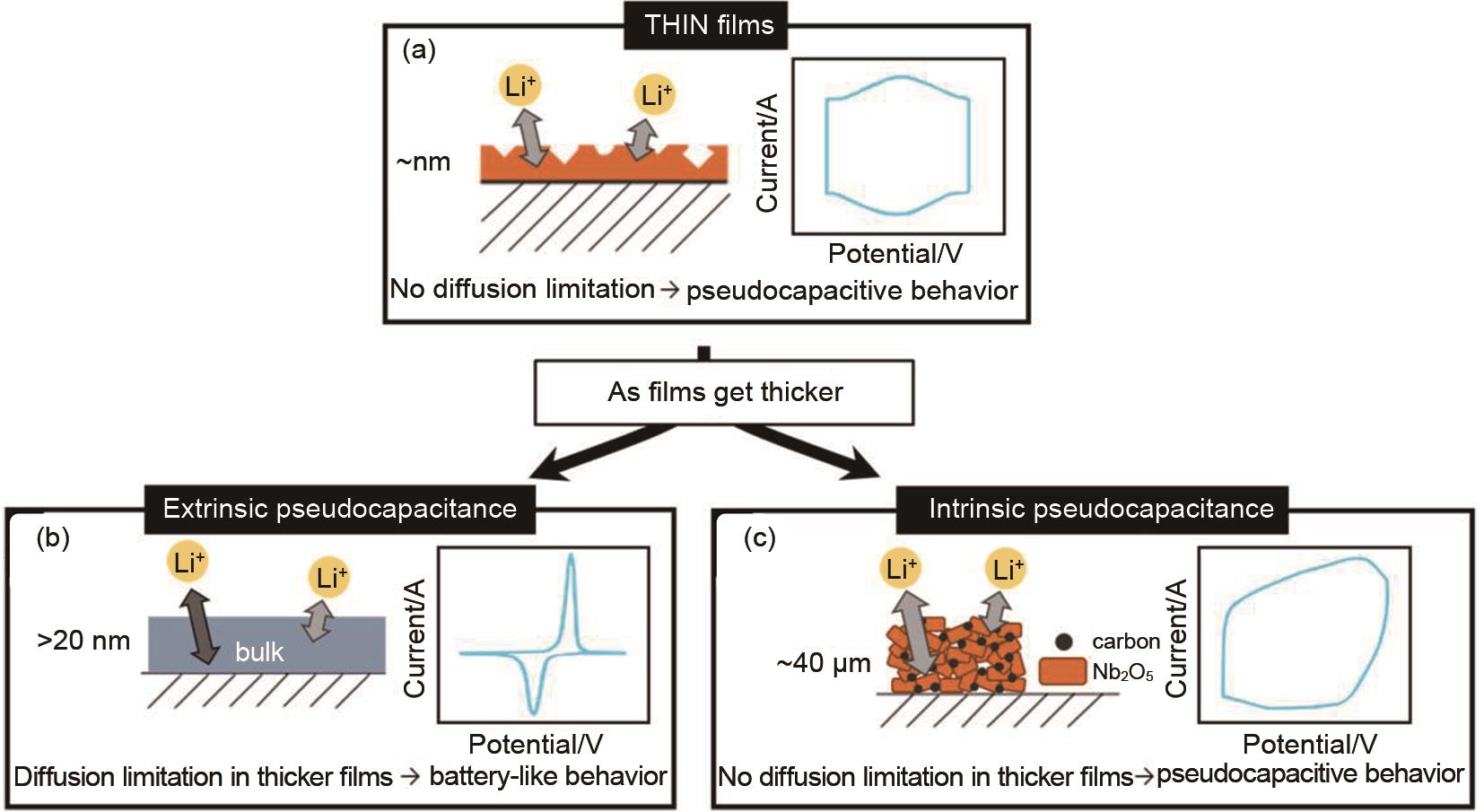
Fig. 5
(a) First-principles modeling schematic of Li+ intercalation into TiO2 (101)[26]; (b) Reorientation of water molecules during geometric optimization, showing initial and final configurations[33]; (c) Pourbaix diagram for the RuO2(110)/water interface; (d) LDOS of Ru and O atoms at br and cus sites[34]; (e) Energy level diagrams of different aromatic isomers[35]"


Fig. 6
(a) Gibbs free energy of Ti3C2T x(T = O, OH) in 1 mol/L H2SO4 electrolyte as a function of H coverage x at different electrode potentials relative to SHE; (b) Faradaic charge (blue, to balance proton transfer), EDL charge (black, due to surface net charge), and total charge (red, net electron transfer number) stored at different electrode potentials[41]; (c) Charge storage per formula unit vs the shift in the potential at the point of zero charge (ΔVPZC) and hydrogen adsorption free energy (ΔGH)[42]; (d) Ion-MXene distance dependence of the experimental specific capacitance[44]; (e) Schematic pictures of the capacitive and pseudocapacitive conditions formed inside the MXene electrode[45]; (f) Pseudocapacitive voltage window size increases with the scaled change in oxidation states during adsorption-induced redox on MXene surfaces[48]"
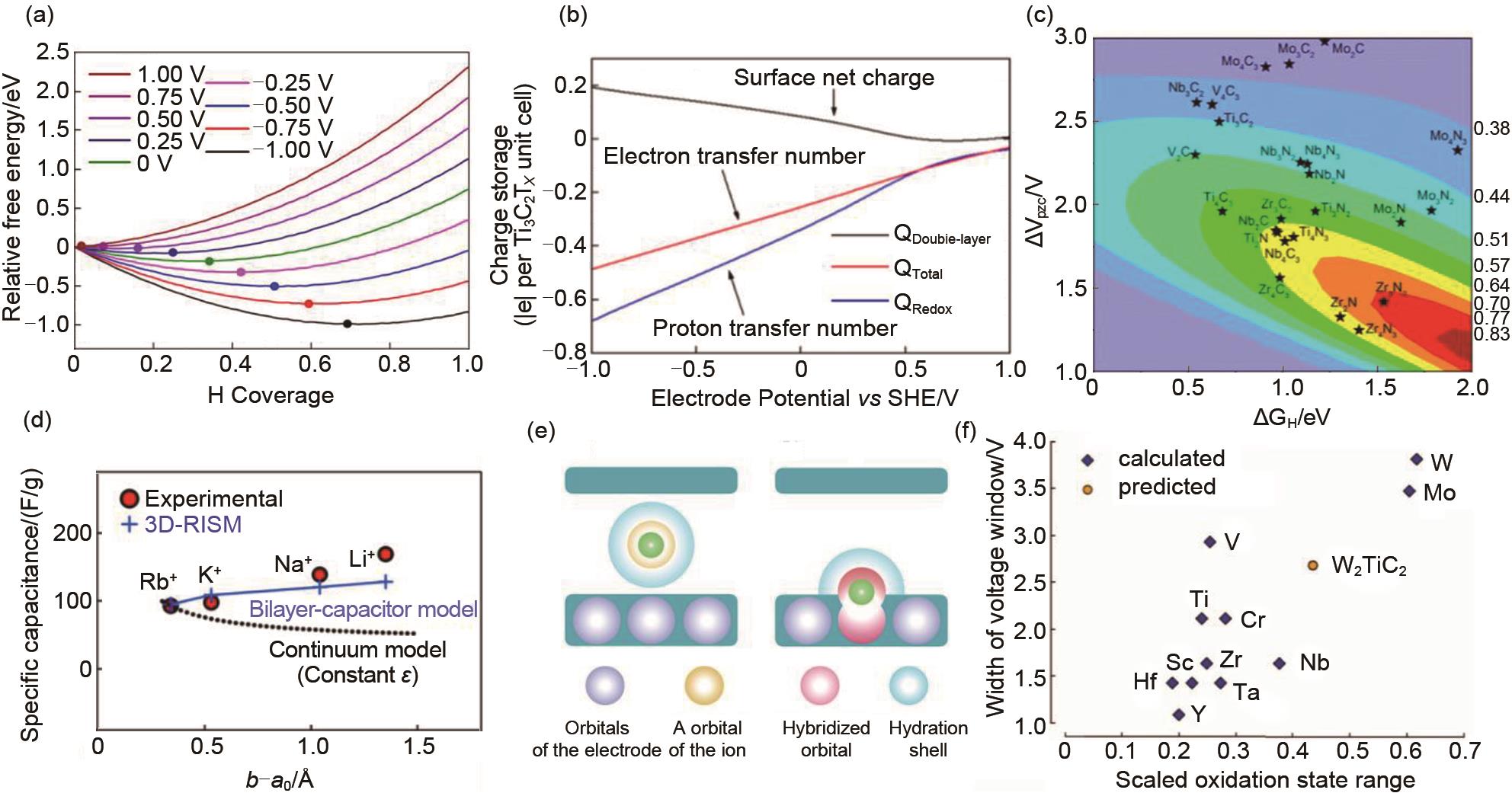

Fig. 7
(a) Snapshots of the ionic arrangements inside one of the Ti3C2F2 electrode poresatdifferent charged state: negatively charged, neutral, and positively charged, respectively[50]; (b) The RDFs forSO42--H2O and H2O-H2O obtained from MD simulations in 6 mol/L H2SO4[51]; (c) Comparison of probability profiles of dipole orientation of water molecules inside P-MXene and 500-MXene layers[52]"

Fig. 8
(a) Schematic of charge/discharge pseudocapacitive process of MoS2[55]; (b) Schematic illustrating the difference between typical pseudocapacitive intercalation and the desolvation-free intercalation observed in Ti3C2T x in WIS electrolytes[63]; (c) In situ XRD map of vacuum-filtered Ti3C2 in DMSO-based electrolyte; (d) MD simulation result for DMSO-based electrolyte, showing a MXene/Li+/DMSO/fast ion transport tunnel[57]"
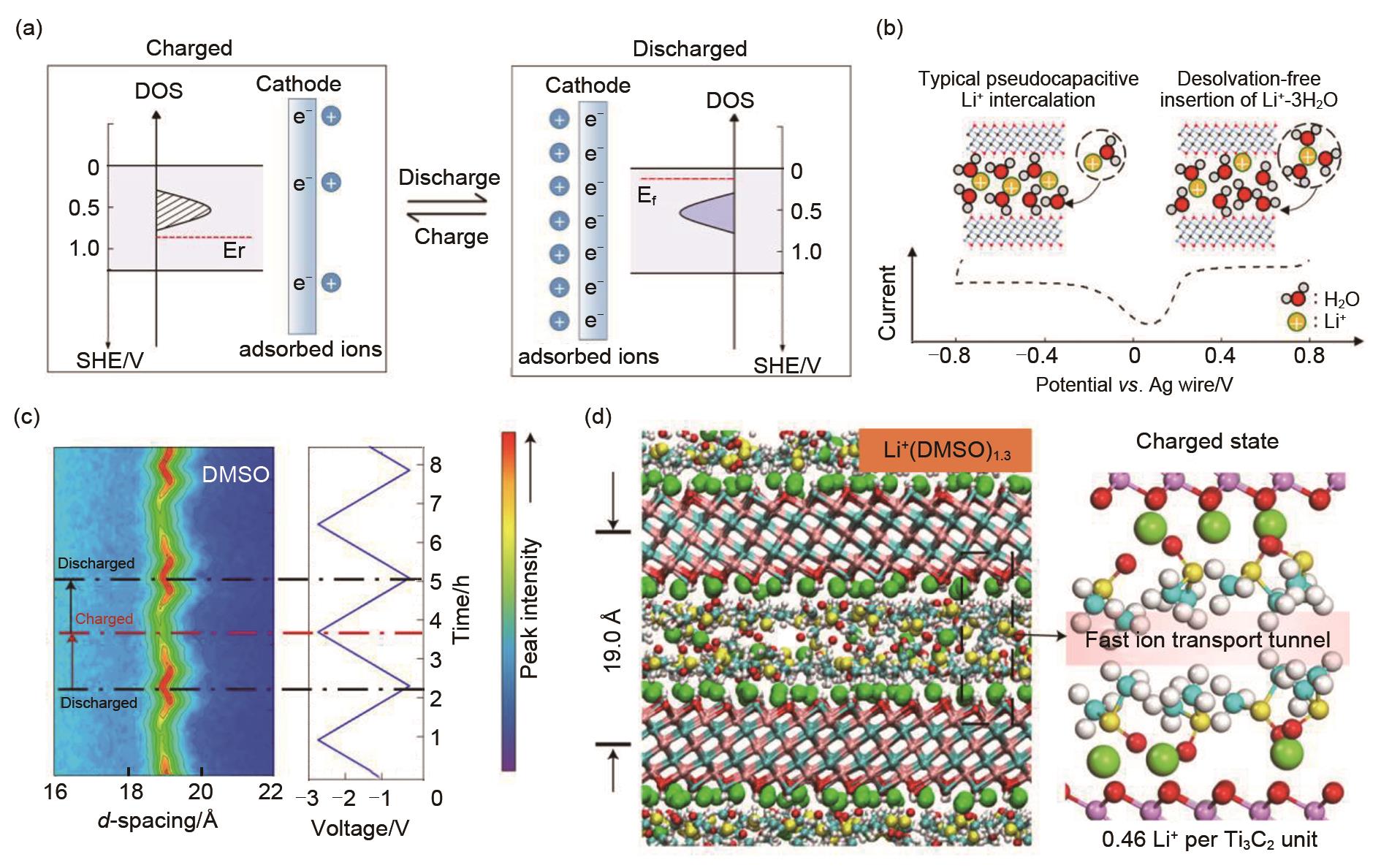
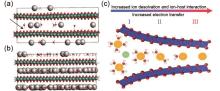
Fig. 9
(a) Snapshot of a ReaxFF GCMC simulation of K+ and H2O intercalation into birnessite. The black arrow shows the region of local wrinkling of the layer due to the inhomogeneity of K+ distribution; (b) Snapshot after the interlayer is fully filled, flattening the wrinkling in the simulation domain[27]; (c) The continuous transition from electrostatic double-layer (I), through a transition region (II) to Faradaic intercalation (III) in nanoconfinement that is driven by the extent of ion solvation and the resulting ion–host material interaction[24]"
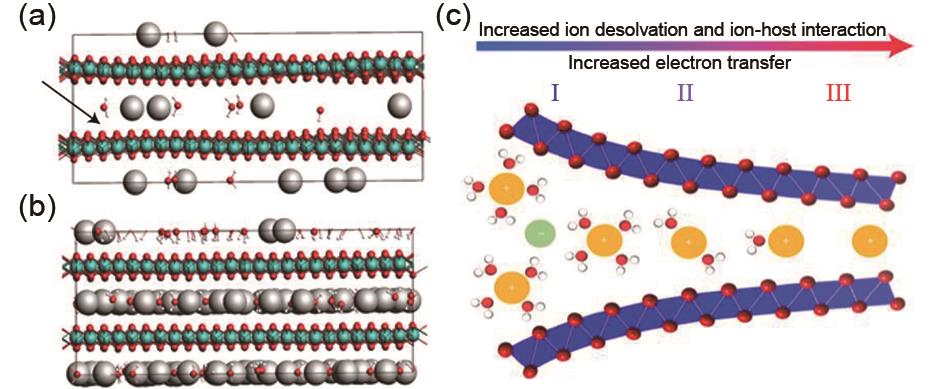
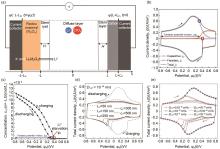
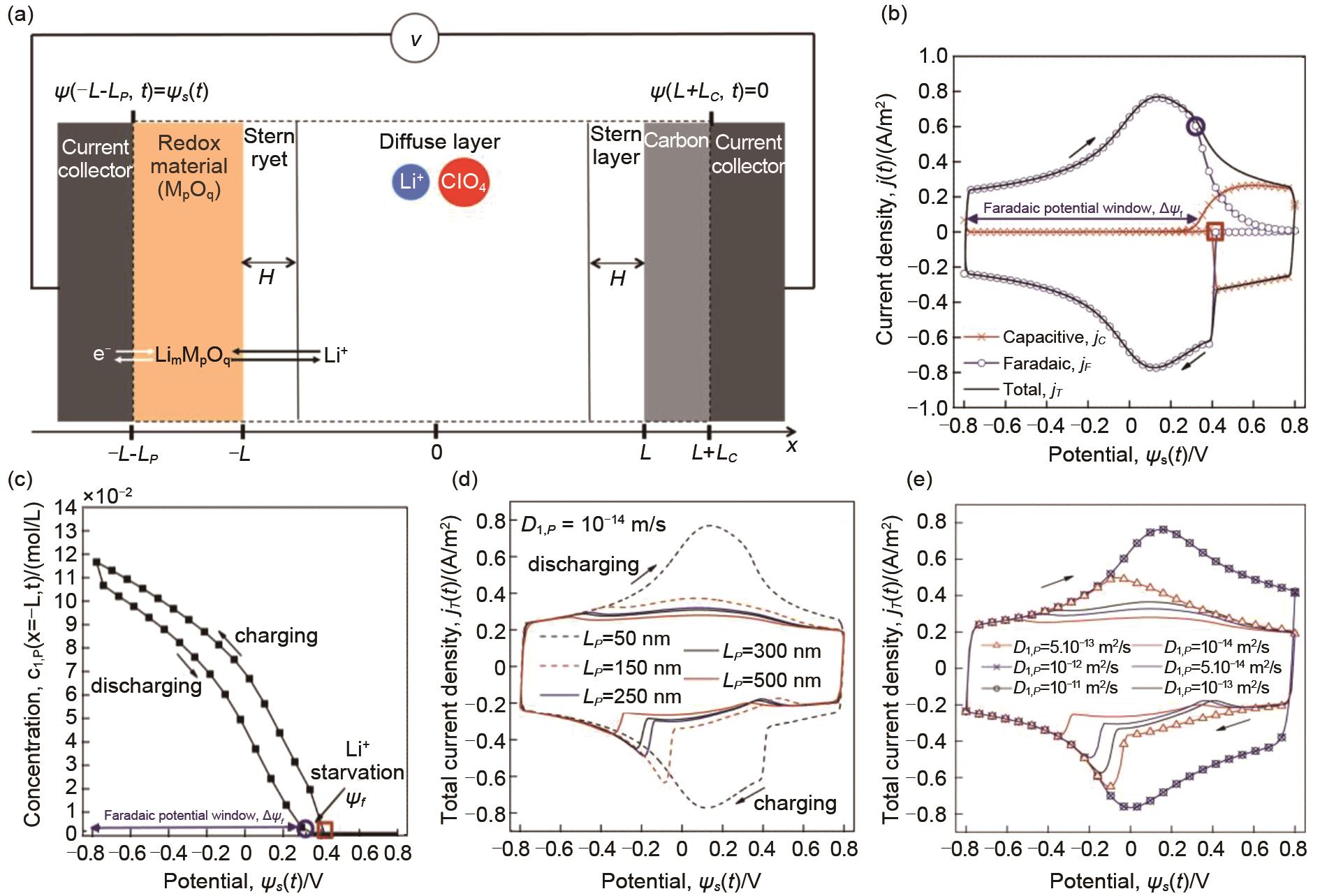
Fig. 10
(a) Schematic of the simulated one-dimensional hybrid pseudocapacitor cell consisting of a redox-active pseudocapacitive electrode and a carbon electrode with LiClO4 electrolyte in PC. The dashed line encloses the computational domain simulated[29]; (b) Li+ concentration evolution at the electrode/electrolyte interface as a function of applied potential under high scan rate (v = 1 V/s); (c) concentration of intercalated Li+ in the pseudocapacitive electrode at the electrode/electrolyte interface as functions ofcell potential s(t) at scan rate v=1 V/s; (d) Variation of total current density with diffusion coefficient; (e) Variation of total current density with Li+ concentration[65]"
| [1] | 陈海生, 李泓, 徐玉杰, 等. 2023年中国储能技术研究进展[J]. 储能科学与技术, 2024, 13(5): 1359-1397. DOI: 10.19799/j.cnki.2095-4239.2024.0441. |
| CHEN H S, LI H, XU Y J, et al. Research progress on energy storage technologies of China in 2023[J]. Energy Storage Science and Technology, 2024, 13(5): 1359-1397. DOI: 10.19799/j.cnki.2095-4239.2024.0441. | |
| [2] | 中国化学会电化学专业委员会. 电化学十大科学问题[J]. 电化学(中英文), 2024, 30(1): 4-14. |
| Chinese Society of Electrochemistry. The top ten scientific questions in electrochemistry[J]. Journal of Electrochemistry, 2024, 30(1): 4-14. | |
| [3] | JIANG Y Q, LIU J P. Definitions of pseudocapacitive materials: A brief review[J]. Energy & Environmental Materials, 2019, 2(1): 30-37. DOI: 10.1002/eem2.12028. |
| [4] | FLEISCHMANN S, MITCHELL J B, WANG R C, et al. Pseudocapacitance: From fundamental understanding to high power energy storage materials[J]. Chemical Reviews, 2020, 120(14): 6738-6782. DOI: 10.1021/acs.chemrev.0c00170. |
| [5] | CHOI C, ASHBY D S, BUTTS D M, et al. Achieving high energy density and high power density with pseudocapacitive materials[J]. Nature Reviews Materials, 2019, 5(1): 5-19. DOI: 10.1038/s41578-019-0142-z. |
| [6] | PARK H W, ROH K C. Recent advances in and perspectives on pseudocapacitive materials for Supercapacitors-a review[J]. Journal of Power Sources, 2023, 557: 232558. DOI: 10.1016/j.jpowsour.2022.232558. |
| [7] | SHAO H, LIN Z F, XU K, et al. Electrochemical study of pseudocapacitive behavior of Ti3C2Tx MXene material in aqueous electrolytes[J]. Energy Storage Materials, 2019, 18: 456-461. DOI: 10.1016/j.ensm.2018.12.017. |
| [8] | COSTENTIN C, PORTER T R, SAVÉANT J M. How do pseudocapacitors store energy? theoretical analysis and experimental illustration[J]. ACS Applied Materials & Interfaces, 2017, 9(10): 8649-8658. DOI: 10.1021/acsami.6b14100. |
| [9] | FOONG Y W, HOSSAIN M S, SUKHOMLINOV S V, et al. Faradaic quantized capacitance as an ideal pseudocapacitive mechanism[J]. The Journal of Physical Chemistry C, 2021, 125(8): 4343-4354. DOI: 10.1021/acs.jpcc.0c07862. |
| [10] | ZHAN C, LIAN C, ZHANG Y, et al. Computational insights into materials and interfaces for capacitive energy storage[J]. Advanced Science, 2017, 4(7): 1700059. DOI: 10.1002/advs. 201700059. |
| [11] | XU K, SHAO H, LIN Z F, et al. Computational insights into charge storage mechanisms of supercapacitors[J]. Energy & Environmental Materials, 2020, 3(3): 235-246. DOI: 10.1002/eem2.12124. |
| [12] | GRAHAME D C. Properties of the electrical double layer at a mercury surface. I. methods of measurement and interpretation of results[J]. Journal of the American Chemical Society, 1941, 63(5): 1207-1215. DOI: 10.1021/ja01850a014. |
| [13] | CONWAY B E, GILEADI E. Kinetic theory of pseudo-capacitance and electrode reactions at appreciable surface coverage[J]. Transactions of the Faraday Society, 1962, 58: 2493-2509. DOI: 10.1039/TF9625802493. |
| [14] | SIMON P, GOGOTSI Y, DUNN B. Where do batteries end and supercapacitors begin?[J]. Science, 2014, 343(6176): 1210-1211. DOI: 10.1126/science.1249625. |
| [15] | WANG J, POLLEUX J, LIM J, et al. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles[J]. The Journal of Physical Chemistry C, 2007, 111(40): 14925-14931. DOI: 10.1021/jp074464w. |
| [16] | TRASATTI S, BUZZANCA G. Ruthenium dioxide: A new interesting electrode material. Solid state structure and electrochemical behaviour[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1971, 29(2): A1-A5. DOI: 10.1016/S0022-0728(71)80111-0. |
| [17] | HU G X, TANG C H, LI C X, et al. The Sol-gel-derived nickel-cobalt oxides with high supercapacitor performances[J]. Journal of the Electrochemical Society, 2011, 158(6): A695. DOI: 10.1149/1.3574021. |
| [18] | YOSHIDA N, YAMADA Y, NISHIMURA S I, et al. Unveiling the origin of unusual pseudocapacitance of RuO2·nH2O from its hierarchical nanostructure by small-angle X-ray scattering[J]. The Journal of Physical Chemistry C, 2013, 117(23): 12003-12009. DOI: 10.1021/jp403402k. |
| [19] | BI S, BANDA H, CHEN M, et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes[J]. Nature Materials, 2020, 19(5): 552-558. DOI: 10.1038/s41563-019-0598-7. |
| [20] | BROUSSE T, BÉLANGER D, LONG J W. To be or not to be pseudocapacitive?[J]. Journal of the Electrochemical Society, 2015, 162(5): A5185-A5189. DOI: 10.1149/2.0201505jes. |
| [21] | COSTENTIN C, SAVÉANT J M. Energy storage: Pseudocapacitance in prospect[J]. Chemical Science, 2019, 10(22): 5656-5666. DOI: 10.1039/C9SC01662G. |
| [22] | GOGOTSI Y, PENNER R M. Energy storage in nanomaterials-capacitive, pseudocapacitive, or battery-like?[J]. ACS Nano, 2018, 12(3): 2081-2083. DOI: 10.1021/acsnano.8b01914. |
| [23] | COME J, AUGUSTYN V, KIM J W, et al. Electrochemical kinetics of nanostructured Nb2O5 electrodes[J]. Journal of the Electrochemical Society, 2014, 161(5): A718-A725. DOI: 10.1149/2.040405jes. |
| [24] | FLEISCHMANN S, ZHANG Y, WANG X P, et al. Continuous transition from double-layer to Faradaic charge storage in confined electrolytes[J]. Nature Energy, 2022, 7(3): 222-228. DOI: 10.1038/s41560-022-00993-z. |
| [25] | JIANG J, CAO D P, JIANG D E, et al. Kinetic charging inversion in ionic liquid electric double layers[J]. The Journal of Physical Chemistry Letters, 2014, 5(13): 2195-2200. DOI: 10.1021/jz5009533. |
| [26] | KANG J, WEI S H, ZHU K, et al. First-principles theory of electrochemical capacitance of nanostructured materials: Dipole-assisted subsurface intercalation of lithium in pseudocapacitive TiO2 anatase nanosheets[J]. The Journal of Physical Chemistry C, 2011, 115(11): 4909-4915. DOI: 10.1021/jp1090125. |
| [27] | BOYD S, GANESHAN K, TSAI W Y, et al. Effects of interlayer confinement and hydration on capacitive charge storage in birnessite[J]. Nature Materials, 2021, 20(12): 1689-1694. DOI: 10.1038/s41563-021-01066-4. |
| [28] | SAMPAIO A M, BI S, SALANNE M, et al. Molecular dynamics simulations of ionic liquids confined into MXenes[J]. Energy Storage Materials, 2024, 70: 103502. DOI: 10.1016/j.ensm. 2024. 103502. |
| [29] | GIRARD H L, WANG H N, D'ENTREMONT A, et al. Physical interpretation of cyclic voltammetry for hybrid pseudocapacitors[J]. The Journal of Physical Chemistry C, 2015, 119(21): 11349-11361. DOI: 10.1021/acs.jpcc.5b00641. |
| [30] | WU Q S, MCDOWELL M T, QI Y. Effect of the electric double layer (EDL) in multicomponent electrolyte reduction and solid electrolyte interphase (SEI) formation in lithium batteries[J]. Journal of the American Chemical Society, 2023, 145(4): 2473-2484. DOI: 10.1021/jacs.2c11807. |
| [31] | DEEBANSOK S, DENG J, LE CALVEZ E, et al. Capacitive tendency concept alongside supervised machine-learning toward classifying electrochemical behavior of battery and pseudocapacitor materials[J]. Nature Communications, 2024, 15: 1133. DOI: 10.1038/s41467-024-45394-w. |
| [32] | WU X, KANG F Y, DUAN W H, et al. Density functional theory calculations: A powerful tool to simulate and design high-performance energy storage and conversion materials[J]. Progress in Natural Science: Materials International, 2019, 29(3): 247-255. DOI: 10.1016/j.pnsc.2019.04.003. |
| [33] | WATANABE E, ROSSMEISL J, BJÖRKETUN M E, et al. Atomic-scale analysis of the RuO2/water interface under electrochemical conditions[J]. The Journal of Physical Chemistry C, 2016, 120(15): 8096-8103. DOI: 10.1021/acs.jpcc.5b12448. |
| [34] | WATANABE E, USHIYAMA H, YAMASHITA K, et al. Charge storage mechanism of RuO2/water interfaces[J]. The Journal of Physical Chemistry C, 2017, 121(35): 18975-18981. DOI: 10. 1021/acs.jpcc.7b02500. |
| [35] | ZHAO Y, WANG X X, WANG N, et al. Unraveling factors leading to high pseudocapacitance of redox-active small aromatics on graphene[J]. The Journal of Physical Chemistry C, 2019, 123(2): 994-1002. DOI: 10.1021/acs.jpcc.8b08348. |
| [36] | LI X L, HUANG Z D, SHUCK C E, et al. MXene chemistry, electrochemistry and energy storage applications[J]. Nature Reviews Chemistry, 2022, 6(6): 389-404. DOI: 10.1038/s41570-022-00384-8. |
| [37] | LU C J, YANG L, YAN B Z, et al. Nitrogen-doped Ti3C2 MXene: Mechanism investigation and electrochemical analysis[J]. Advanced Functional Materials, 2020, 30(47): 2000852. DOI: 10.1002/adfm.202000852. |
| [38] | BO Z, CHEN Y C, YU Q, et al. Unveiling the energy storage mechanism of MXenes under acidic conditions through transitions of surface functionalizations[J]. The Journal of Physical Chemistry C, 2024, 128(6): 2352-2361. DOI: 10.1021/acs.jpcc.3c06956. |
| [39] | CRAMER C J, TRUHLAR D G. Implicit solvation models: Equilibria, structure, spectra, and dynamics[J]. Chemical Reviews, 1999, 99(8): 2161-2200. DOI: 10.1021/cr960149m. |
| [40] | ZHAN C, JIANG D E. Understanding the pseudocapacitance of RuO2 from joint density functional theory[J]. Journal of Physics Condensed Matter, 2016, 28(46): 464004. DOI: 10.1088/0953-8984/28/46/464004. |
| [41] | ZHAN C, NAGUIB M, LUKATSKAYA M, et al. Understanding the MXene pseudocapacitance[J]. The Journal of Physical Chemistry Letters, 2018, 9(6): 1223-1228. DOI: 10.1021/acs.jpclett.8b00200. |
| [42] | ZHAN C, SUN W W, KENT P R C, et al. Computational screening of MXene electrodes for pseudocapacitive energy storage[J]. The Journal of Physical Chemistry C, 2019, 123(1): 315-321. DOI: 10. 1021/acs.jpcc.8b11608. |
| [43] | KOVALENKO A, HIRATA F. Self-consistent description of a metal-water interface by the Kohn-Sham density functional theory and the three-dimensional reference interaction site model[J]. The Journal of Chemical Physics, 1999, 110(20): 10095-10112. DOI: 10.1063/1.478883. |
| [44] | SUGAHARA A, ANDO Y, KAJIYAMA S, et al. Negative dielectric constant of water confined in nanosheets[J]. Nature Communications, 2019, 10: 850. DOI: 10.1038/s41467-019-08789-8. |
| [45] | ANDO Y, OKUBO M, YAMADA A, et al. Capacitive versus pseudocapacitive storage in MXene[J]. Advanced Functional Materials, 2020, 30(47): 2000820. DOI: 10.1002/adfm. 202000820. |
| [46] | HARUYAMA J, IKESHOJI T, OTANI M. Electrode potential from density functional theory calculations combined with implicit solvation theory[J]. Physical Review Materials, 2018, 2(9): 095801. DOI: 10.1103/PhysRevMaterials.2.095801. |
| [47] | CHEN G L, XIE Y Y, TANG Y, et al. Unraveling the role of metal vacancy sites and doped nitrogen in enhancing pseudocapacitance performance of defective MXene[J]. Small, 2024, 20(12): 2307408. DOI: 10.1002/smll.202307408. |
| [48] | GOFF J M, MARQUES DOS SANTOS VIEIRA F, KEILBART N D, et al. Predicting the pseudocapacitive windows for MXene electrodes with voltage-dependent cluster expansion models[J]. ACS Applied Energy Materials, 2021, 4(4): 3151-3159. DOI: 10.1021/acsaem.0c02910. |
| [49] | RAPAPORT D C. Molecular dynamics simulation[J]. Computing in Science & Engineering, 1999, 1(1): 70-71. DOI: 10.1109/5992. 743625. |
| [50] | XU K, LIN Z F, MERLET C, et al. Tracking ionic rearrangements and interpreting dynamic volumetric changes in two-dimensional metal carbide supercapacitors: A molecular dynamics simulation study[J]. ChemSusChem, 2018, 11(12): 1892-1899. DOI: 10. 1002/cssc.201702068. |
| [51] | XU T Z, WANG D, ZHANG M R, et al. Ultralow-temperature (≤ -80 ℃) proton pseudocapacitor with high power-energy density enabled by tailored proton-rich electrolyte and electrode[J]. Advanced Functional Materials, 2024, 34(48): 2408465. DOI: 10.1002/adfm.202408465. |
| [52] | SHAO H, XU K, WU Y C, et al. Unraveling the charge storage mechanism of Ti3C2Tx MXene electrode in acidic electrolyte[J]. ACS Energy Letters, 2020, 5(9): 2873-2880. DOI: 10.1021/acsenergylett.0c01290. |
| [53] | ZENG L, TAN X, JI X Y, et al. Constant charge method or constant potential method: Which is better for molecular modeling of electrical double layers?[J]. Journal of Energy Chemistry, 2024, 94: 54-60. DOI: 10.1016/j.jechem.2024.02.043. |
| [54] | MO T M, WANG Z X, ZENG L, et al. Energy storage mechanism in supercapacitors with porous graphdiynes: Effects of pore topology and electrode metallicity[J]. Advanced Materials, 2023, 35(33): 2301118. DOI: 10.1002/adma.202301118. |
| [55] | ZHANG B, JI X, XU K, et al. Unraveling the different charge storage mechanism in T and H phases of MoS2[J]. Electrochimica Acta, 2016, 217: 1-8. DOI: 10.1016/j.electacta.2016.09.059. |
| [56] | CAR R, PARRINELLO M. Unified approach for molecular dynamics and density-functional theory[J]. Physical Review Letters, 1985, 55(22): 2471-2474. DOI: 10.1103/PhysRevLett. 55. 2471. |
| [57] | WANG X H, MATHIS T S, LI K, et al. Influences from solvents on charge storage in titanium carbide MXenes[J]. Nature Energy, 2019, 4(3): 241-248. DOI: 10.1038/s41560-019-0339-9. |
| [58] | TANG B, FANG Y G, ZHU S, et al. Tuning hydrogen bond network connectivity in the electric double layer with cations[J]. Chemical Science, 2024, 15(19): 7111-7120. DOI: 10.1039/D3SC06904D. |
| [59] | JIN T, LI H X, LI Y, et al. Intercalation pseudocapacitance in flexible and self-standing V2O3 porous nanofibers for high-rate and ultra-stable K ion storage[J]. Nano Energy, 2018, 50: 462-467. DOI: 10.1016/j.nanoen.2018.05.056. |
| [60] | MU X P, WANG D S, DU F, et al. Revealing the pseudo-intercalation charge storage mechanism of MXenes in acidic electrolyte[J]. Advanced Functional Materials, 2019, 29(29): 1902953. DOI: 10.1002/adfm.201902953. |
| [61] | SUN Y, ZHAN C, KENT P R C, et al. Proton redox and transport in MXene-confined water[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 763-770. DOI: 10.1021/acsami.9b18139. |
| [62] | SONG H H, JIANG D E. First principles insights into stability of defected MXenes in water[J]. Nanoscale, 2023, 15(39): 16010-16015. DOI: 10.1039/D3NR02538A. |
| [63] | WANG X H, MATHIS T S, SUN Y, et al. Titanium carbide MXene shows an electrochemical anomaly in water-in-salt electrolytes[J]. ACS Nano, 2021, 15(9): 15274-15284. DOI: 10.1021/acsnano. 1c06027. |
| [64] | LIU Y, YU P P, WU Y, et al. The DFT-ReaxFF hybrid reactive dynamics method with application to the reductive decomposition reaction of the TFSI and DOL electrolyte at a lithium-metal anode surface[J]. The Journal of Physical Chemistry Letters, 2021, 12(4): 1300-1306. DOI: 10.1021/acs.jpclett.0c03720. |
| [65] | GIRARD H L, WANG H N, D'ENTREMONT A L, et al. Enhancing faradaic charge storage contribution in hybrid pseudocapacitors[J]. Electrochimica Acta, 2015, 182: 639-651. DOI: 10.1016/j.electacta.2015.09.070. |
| [66] | GIRARD H L, DUNN B, PILON L. Simulations and interpretation of three-electrode cyclic voltammograms of pseudocapacitive electrodes[J]. Electrochimica Acta, 2016, 211: 420-429. DOI: 10.1016/j.electacta.2016.06.066. |
| [67] | LIN H, TRUHLAR D G. QM/MM: What have we learned, where are we, and where do we go from here?[J]. ChemInform, 2007, 38(22): 185-199. DOI: 10.1002/chin.200722224. |
| [68] | KOCER E, KO T W, BEHLER J. Neural network potentials: A concise overview of methods[J]. Annual Review of Physical Chemistry, 2022, 73: 163-186. DOI: 10.1146/annurev-physchem-082720-034254. |
| [69] | XIE S R, RUPP M, HENNIG R G. Ultra-fast interpretable machine-learning potentials[J]. NPJ Computational Materials, 2023, 9: 162. DOI: 10.1038/s41524-023-01092-7. |
| [70] | 邓斌, 华海明, 张与之, 等. 深度势能方法及其在电化学储能材料中的应用[J]. 储能科学与技术, 2024, 13(9): 2884-2906. DOI: 10. 19799/j.cnki.2095-4239.2024.0699. |
| DENG B, HUA H M, ZHANG Y Z, et al. Deep potential model: Applications and insights for electrochemical energy storage materials[J]. Energy Storage Science and Technology, 2024, 13(9): 2884-2906. DOI: 10.19799/j.cnki.2095-4239.2024.0699. | |
| [71] | WANG Z X, WU T Z, ZENG L, et al. Machine learning relationships between nanoporous structures and electrochemical performance in MOF supercapacitors[J]. Advanced Materials, 2025, 37(15): 2500943. DOI: 10.1002/adma. 202500943. |
| [72] | BOOTA M, ANASORI B, VOIGT C, et al. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene)[J]. Advanced Materials, 2016, 28(7): 1517-1522. DOI: 10.1002/adma. 201504705. |
| [73] | ZE H J, FAN X T, YANG Z L, et al. Deciphering the competitive charge storage chemistry of metal cations and protons in aqueous MnO2-based supercapacitors[J]. Journal of the American Chemical Society, 2025, 147(11): 9620-9628. DOI: 10. 1021/jacs.4c17458. |
| [1] | Guobing ZHOU, Shenzhen XU. Progress of theoretical studies on the formation and growth mechanisms of solid electrolyte interphase at lithium metal anodes [J]. Energy Storage Science and Technology, 2024, 13(9): 3150-3160. |
| [2] | Chenyang ZHAO, Xiaokun YU, Yubing TAO. Preparation and characterization of modified CuO nanoparticles/n-octadecane phase change material [J]. Energy Storage Science and Technology, 2024, 13(6): 1786-1793. |
| [3] | Heqing TIAN, Zhaoyang KOU, Junjie ZHOU, Yinsheng YU. Molecular dynamics simulation of structure and thermal properties of LiCl-KCl molten salt nanofluids [J]. Energy Storage Science and Technology, 2023, 12(3): 654-660. |
| [4] | Yuting ZHU, Gongqin YAN, Yuqian LIN. Electrochemical properties and First-principles study of MoS2/rGO composite [J]. Energy Storage Science and Technology, 2023, 12(3): 698-709. |
| [5] | Huimin ZHANG, Jing WANG, Yibo WANG, Jiaxin ZHENG, Jingyi QIU, Gaoping CAO, Hao ZHANG. Multiscale modeling of the SEI of lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(2): 366-382. |
| [6] | Dianwei FU, Cancan ZHANG, Heya NA, Guoqiang WANG, Yuting WU, Yuanwei LU. Review of the molecular dynamics of molten salt thermal physical properties [J]. Energy Storage Science and Technology, 2023, 12(12): 3873-3882. |
| [7] | Liangtao XIONG, Jifen WANG, Huaqing XIE, Xuelai ZHANG. Effect of vacancy defects on thermal conductivity of single-layer graphene by molecular dynamics [J]. Energy Storage Science and Technology, 2022, 11(5): 1322-1330. |
| [8] | Peiping YU, Liang XU, Bingyun MA, Qintao SUN, Hao YANG, Yue LIU, Tao CHENG. Multiscale simulation of a solid electrolyte interphase [J]. Energy Storage Science and Technology, 2022, 11(3): 921-928. |
| [9] | Min'an YANG, Ning CHEN, Bo WANG, Qian ZHANG, Jingpei CHEN, Hailei ZHAO, Fushen LI. Gene law about cycle stability of cathode material for lithium-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(2): 462-469. |
| [10] | ZHANG Xuelai, WANG Xuzhe, WANG Jifen, XU Xiaofeng, HUA Weisan, FANG Manting. Molecular dynamics simulation of phase transformation process of n-tetradecane [J]. Energy Storage Science and Technology, 2019, 8(5): 874-879. |
| [11] | YU Jiapeng, CHENG Xiaomin, LI Yuanyuan, LI Bei, XU Hong. Molecular dynamics simulation of thermodynamic properties of Mg-Cu alloys [J]. Energy Storage Science and Technology, 2019, 8(4): 772-777. |
| [12] | CHEN Bingbing, ZHAO Jinwen, MA Jun, CUI Guanglei. Relationship of ion transport and pressure in PEO/LITFSI solid electrolytes [J]. Energy Storage Science and Technology, 2018, 7(3): 431-436. |
| [13] | ZHENG Chao, LI Linyan, CHEN Xuedan, YU Xuewen, GU Yingzhan, WU Yihuan, DING Sheng, PAN Guolin, ZHOU Zhou, LIU Qiuxiang, CHEN Kuan, YUAN Jun, YANG Bin, QIAO Zhijun, FU Guansheng, RUAN Dianbo. Review of selected 100 recent papers for supercapacitors(Jul. 1,2017 to Dec. 15,2017) [J]. Energy Storage Science and Technology, 2018, 7(1): 20-. |
| [14] | ZHENG Chao, CHEN Xuedan, GU Yingzhan, WU Yihuan, DING Sheng, PAN Guolin, ZHOU Zhou, LI Linyan, LIU Qiuxiang, YU Xuewen, CHEN Kuan, YUAN Jun, YAN Bin, QIAO Zhijun, FU Guansheng, RUAN Dianbo. Review of selected 100 recent papers for supercapacitors(Oct. 1,2016 to Jun. 30,2017) [J]. Energy Storage Science and Technology, 2017, 6(5): 1128-1144. |
| [15] | NI Haiou, SUN Ze, LU Guimin, YU Jianguo. Molecular dynamics simulation of structure and physical properties of NaNO3-KNO3-NaNO2 ternary phase-change molten salts [J]. Energy Storage Science and Technology, 2017, 6(4): 669-674. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
