储能科学与技术 ›› 2023, Vol. 12 ›› Issue (5): 1348-1363.doi: 10.19799/j.cnki.2095-4239.2023.0257
• 喜迎东北大学建校百年-储能电池关键材料与循环技术专刊 • 上一篇 下一篇
收稿日期:2023-04-21
修回日期:2023-05-09
出版日期:2023-05-05
发布日期:2023-05-29
通讯作者:
刘朝孟
E-mail:2171602@stu.neu.edu.cn;liuzhaomeng@smm.neu.edu.cn
作者简介:李尚倬(1999—),男,硕士研究生,研究方向为电池正极材料,E-mail:2171602@stu.neu.edu.cn;
基金资助:
Shangzhuo LI( ), Yutong LONG, Zhaomeng LIU(
), Yutong LONG, Zhaomeng LIU( ), Xuanwen GAO, Wenbin LUO
), Xuanwen GAO, Wenbin LUO
Received:2023-04-21
Revised:2023-05-09
Online:2023-05-05
Published:2023-05-29
Contact:
Zhaomeng LIU
E-mail:2171602@stu.neu.edu.cn;liuzhaomeng@smm.neu.edu.cn
摘要:
钾离子电池(PIBs)由于钾金属资源丰富、成本低廉、环境友好及能量密度高等优点,已成为替代锂离子电池(LIBs)的理想新型储能体系。尽管近年来PIBs在负极领域的研究已经取得了显著进展,但正极材料的研究缓慢,其设计和应用面临可逆比容量低、循环稳定性差、能量密度不理想等问题。因此,发现和设计正极材料对构建用于实际应用的钾离子(K+)电池至关重要。由于聚阴离子材料在LIBs和钠离子电池(NIBs)中的成功应用,近年来,人们也将研究集中于PIBs的聚阴离子材料。聚阴离子材料具有氧化还原电位高、发生有利的感应效应、安全性高、热稳定性和结构稳定性良好等优点,可以实现较为稳定的容量存储,但是其可逆容量低、导电性差等问题仍需解决。本篇综述针对钾离子电池聚阴离子正极材料的研究进行了综述和讨论,以探究聚阴离子正极材料发展设计潜力和研究空间为目的,集中讨论了磷酸盐、氟磷酸盐、焦磷酸盐、硫酸盐类材料的机理和结构的发展现状,总结了当前聚阴离子类正极材料设计的主要理念,并对聚阴离子正极材料的改性研究提出了一些建议和前景。
中图分类号:
李尚倬, 龙禹彤, 刘朝孟, 高宣雯, 骆文彬. 钾离子电池聚阴离子正极材料的研究进展[J]. 储能科学与技术, 2023, 12(5): 1348-1363.
Shangzhuo LI, Yutong LONG, Zhaomeng LIU, Xuanwen GAO, Wenbin LUO. Advances toward polyanionic cathode materials for potassium-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(5): 1348-1363.

图2
(a) K3V2(PO4)3/C的合成机理示意图[36];(b) K3V2(PO4)3/C的N2 吸附-解吸等温线(插图:K3V2(PO4)3/C的孔径分布曲线)[36];(c) K3V2(PO4)3/C捆扎线制备工艺及机理合成图[39];(d) K3V2(PO4)3/C捆扎线在700~900℃的XRD图[39];(e) 100 mAh/g充放电时的原位XRD图和电压叠加曲线图[39];(f) K3V2(PO4)3/C捆扎线在1000和2000 mA/g下的长寿命循环性能[39];(g) 对K3V2(PO4)3 结构的SXRD进行Rietveld细化图[43];(h) K3V2(PO4)3 的单位结构随CV曲线在1.0~4.0 V范围内的演化[43]"
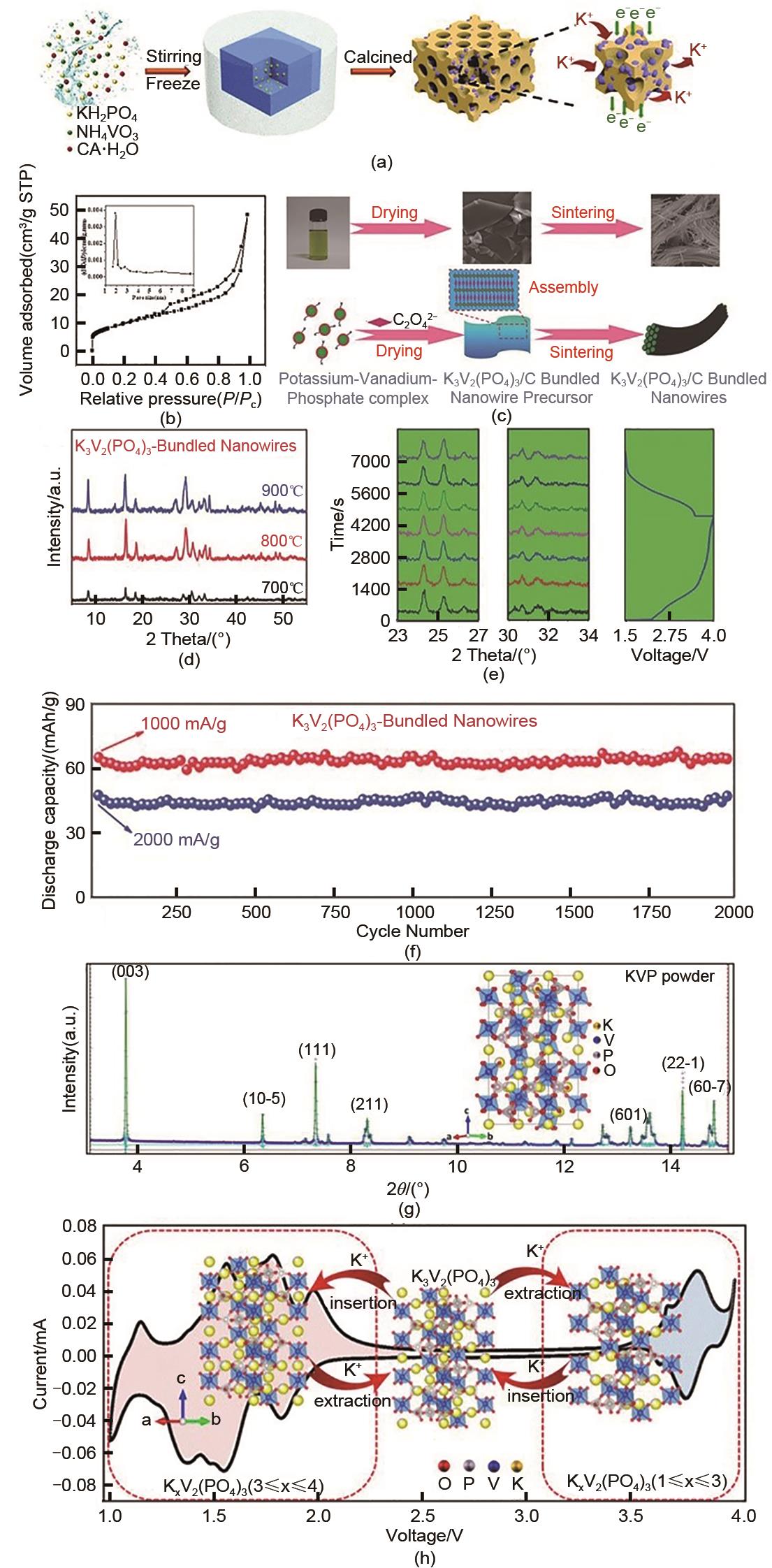
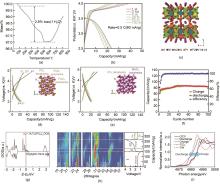
图3
(a) K3V3(PO4)4·H2O的TGA曲线[44];(b) K3V3(PO4)4·H2O第1~100次循环的充放电曲线[44];(c) 平行于(a) x-z 基面[001]K3V3(PO4)4·H2O电位K+ 扩散通道的透视图[44];(d) 当电流密度为7.1 mA/g时KFePO4 的充放电曲线和晶体结构示意图[48];(e) 当电流密度为7.1 mA/g时KMnPO4 的充放电曲线和晶体结构示意图[48];(f) KFePO4/C的N2 吸附-解吸等温线(插图: KFePO4/C的孔径分布曲线)[49];(g) K3Ti2(PO4)3 的DOS图[53];(h) KTP/C的结构演变:第一次充放电和第二次放电开始时操作XRD数据的二维图像[53];(i) 在OCV、放电结束(1 V)和充电(4 V)下测量的KTP/C电极和参比(TiO2 和TiN)的Ti K-边缘的XANES光谱[53]"

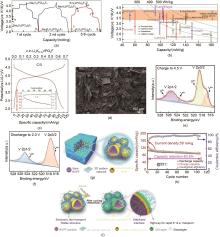
图6
(a) Na3V2(PO4)2F3 钠脱出和随后的钾嵌入/脱出的电压分布[65];(b) 目前报道的不同阴极对制备的K3V2(PO4)2F3 电压和容量的影响[65];(c) Li x K0.15VPO4F在0.2 C时的典型充放电曲线(插图展示了1 C速率下循环能力测试中的容量保持和库仑效率)[67];(d) K1+δ VOPO4F的SEM图[68];(e) K1+δ VOPO4F充电至4.5 V的非原位XPS[68];(f) K1+δ VOPO4F放电至2.0 V的非原位XPS[68];(g) KVPO4F@3DC设计示意图[69];(h) 当电流密度为50 mA/g时,KVPF@3DC在高温(55 ℃)下的循环性能[69];(i) KVPO4F@3DC高温反复充放电过程中界面变化示意图[69]"

表1
近年钾离子电池聚阴离子类正极材料电化学性能与空间构型[46, 54, 62, 65, 71]"
| Materials | Structure | Theoretical capacity /(mAh/g) | Actual capacity /(mAh/g) | Average dischargepotential (vs. K+/K) /V |
|---|---|---|---|---|
| KVPO4F | KTiOPO4(KTP)-type orthorhombic (Pna21) | 131 | 90 | 4.13 |
| KVOPO4 | KTP-type orthorhombic (Pna21) | 133 | 80 | 4 |
| KVP2O7 | KAlP2O7-type monoclinic (P21/c) | 102 | 61 | 4.15 |
| K3V2(PO4)3 | Unknown | 106 | 54 | 3.5 |
| K3V2(PO4)2F3 | Orthorhombic (Cmcm) | 115 | 104 | 3.7 |
| Amorphous-FePO4 | — | 178 | 156 | 2.64 |
| Orthorhombic KFeSO4F | KTP-type orthorhombic (Pna21) | 128 | 100 | 3.6 |
| Monoclinic KFeSO4F | Layered monoclinic (C2/c) | 128 | 50 | 3.5(vs. Li) |
| 1 | ZHANG Q, WANG Z J, ZHANG S L, et al. Cathode materials for potassium-ion batteries: Current status and perspective[J]. Electrochemical Energy Reviews, 2018, 1(4): 625-658. |
| 2 | KOMABA S, HASEGAWA T, DAHBI M, et al. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors[J]. Electrochemistry Communications, 2015, 60: 172-175. |
| 3 | LIU Z, WANG J, JIA X, et al. Graphene armored with a crystal carbon shell for ultrahigh-performance potassium ion batteries and aluminum batteries[J]. ACS Nano, 2019, 13(9): 10631-10642. |
| 4 | LIU Z, WANG J, DING H, et al. Carbon nanoscrolls for aluminum battery[J]. ACS Nano, 2018, 12(8): 8456-8466. |
| 5 | JIAN Z, LUO W, JI X. Carbon electrodes for K-ion batteries[J]. J Am Chem Soc, 2015, 137(36): 11566-11569. |
| 6 | PRAMUDITA J C, SEHRAWAT D, GOONETILLEKE D, et al. An initial review of the status of electrode materials for potassium‐ion batteries[J]. Advanced Energy Materials, 2017, 7(24): 1602911. |
| 7 | KIM H, SEO D H, KIM J C, et al. Investigation of potassium storage in layered P3-type K0.5MnO2 cathode[J]. Advanced Materials, 2017, 29(37): 1702480. |
| 8 | NAVEEN N, PARK W B, HAN S C, et al. Reversible K+-insertion/deinsertion and concomitant Na+-redistribution in P'3-Na0.52CrO2 for high-performance potassium-ion battery cathodes[J]. Chemistry of Materials, 2018, 30(6): 2049-2057. |
| 9 | ZHU Y H, YANG X, SUN T, et al. Recent progresses and prospects of cathode materials for non-aqueous potassium-ion batteries[J]. Electrochemical Energy Reviews, 2018, 1(4): 548-566. |
| 10 | WANG B Q, HAN Y, WANG X, et al. Prussian blue analogs for rechargeable batteries[J]. iScience, 2018, 3: 110-133. |
| 11 | ZHANG Z Y, LI M L, GAO Y, et al. Fast potassium storage in hierarchical Ca0.5Ti2(PO4)3@C microspheres enabling high-performance potassium-ion capacitors[J]. Advanced Functional Materials, 2018, 28(36): 1802684. |
| 12 | MASESE T, YOSHII K, KATO M, et al. A high voltage honeycomb layered cathode framework for rechargeable potassium-ion battery: P2-type K2/3Ni1/3Co1/3Te1/3O2[J]. Chemical Communications (Cambridge, England), 2019, 55(7): 985-988. |
| 13 | ZHANG H Y, XI K Y, JIANG K Z, et al. Enhanced K-ion kinetics in a layered cathode for potassium ion batteries[J]. Chemical Communications (Cambridge, England), 2019, 55(55): 7910-7913. |
| 14 | WU X Y, LEONARD D P, JI X L. Emerging non-aqueous potassium-ion batteries: Challenges and opportunities[J]. Chemistry of Materials, 2017, 29(12): 5031-5042. |
| 15 | 任重民, 王斌, 陈帅帅, 等. 层状正极材料力学劣化及改善措施[J]. 储能科学与技术, 2022, 11(3): 948-956 |
| REN Z M, WANG B, CHEN S S, et al. Mechanics-induced degradation on layer-structured cathodes and remedies to address it[J]. Energy Storage Science and Technology, 2022, 11(3): 948-956 | |
| 16 | XUE L G, LI Y T, LÜ X J, et al. Low-cost high-energy potassium cathode[J]. Journal of the American Chemical Society, 2017, 139(6): 2164-2167. |
| 17 | BIE X F, KUBOTA K, HOSAKA T, et al. A novel K-ion battery: Hexacyanoferrate(ii)/graphite cell[J]. Journal of Materials Chemistry A, 2017, 5(9): 4325-4330. |
| 18 | MANTHIRAM A, GOODENOUGH J B. Vanishing of superconductivity at a transition from itinerant-electron to small-polaron conduction in nominal Bi4– xPbx(Sr3Ca)Ca2– xYxCu4O16[J]. Applied Physics Letters, 1988, 53(26): 2695-2697. |
| 19 | NISHIMURA S I, NAKAMURA M, NATSUI R, et al. New lithium iron pyrophosphate as 3.5 V class cathode material for lithium ion battery[J]. Journal of the American Chemical Society, 2010, 132(39): 13596-13597. |
| 20 | RECHAM N, CHOTARD J N, DUPONT L, et al. A 3.6 V lithium-based fluorosulphate insertion positive electrode for lithium-ion batteries[J]. Nature Materials, 2010, 9(1): 68-74. |
| 21 | BARPANDA P, LANDER L, NISHIMURA S I, et al. Polyanionic insertion materials for sodium-ion batteries[J]. Advanced Energy Materials, 2018, 8(17): 1703055. |
| 22 | GOVER R K B, BRYAN A, BURNS P, et al. The electrochemical insertion properties of sodium vanadium fluorophosphate, Na3V2(PO4)2F3[J]. Solid State Ionics, 2006, 177(17/18): 1495-1500. |
| 23 | BIANCHINI M, BRISSET N, FAUTH F, et al. Na3V2(PO4)2F3 revisited: A high-resolution diffraction study[J]. Chemistry of Materials, 2014, 26(14): 4238-4247. |
| 24 | BIANCHINI M, FAUTH F, BRISSET N, et al. Comprehensive investigation of the Na3V2(PO4)2F3-NaV2(PO4)2F3 system by operando high resolution synchrotron X-ray diffraction[J]. Chemistry of Materials, 2015, 27(8): 3009-3020. |
| 25 | MU J J, LIU Z M, LAI Q S, et al. An industrial pathway to emerging presodiation strategies for increasing the reversible ions in sodium-ion batteries and capacitors[J]. Energy Materials, 2022, 2(6): 200043. |
| 26 | BARPANDA P, YE T, NISHIMURA S I, et al. Sodium iron pyrophosphate: A novel 3.0V iron-based cathode for sodium-ion batteries[J]. Electrochemistry Communications, 2012, 24: 116-119. |
| 27 | BARPANDA P, OYAMA G, NISHIMURA S I, et al. A 3.8-V earth-abundant sodium battery electrode[J]. Nature Communications, 2014, 5(1): 1-8. |
| 28 | CHEN M Z, CORTIE D, HU Z, et al. A novel graphene oxide wrapped Na2Fe2(SO4)3/C cathode composite for long life and high energy density sodium-ion batteries[J]. Advanced Energy Materials, 2018, 8(27): 1800944. |
| 29 | CHEN M Z, CHEN L N, HU Z, et al. Carbon-coated Na3.32Fe2.34(P2O7)2 cathode material for high-rate and long-life sodium-ion batteries[J]. Advanced Materials, 2017, 29(21): 1605535. |
| 30 | CHEN M Z, HUA W B, XIAO J, et al. NASICON-type air-stable and all-climate cathode for sodium-ion batteries with low cost and high-power density[J]. Nature Communications, 2019, 10: 1480. |
| 31 | YAN G C, MARIYAPPAN S, ROUSSE G, et al. Higher energy and safer sodium ion batteries via an electrochemically made disordered Na3V2(PO4)2F3 material[J]. Nature Communications, 2019, 10(1): 1-12. |
| 32 | KIM J, YOON G, KIM H, et al. Na3V(PO4)2: A new layered-type cathode material with high water stability and power capability for Na-ion batteries[J]. Chemistry of Materials, 2018, 30(11): 3683-3689. |
| 33 | HE G, KAN W H, MANTHIRAM A. Delithiation/lithiation behaviors of three polymorphs of LiVOPO4[J]. Chemical Communications, 2018, 54(94): 13224-13227. |
| 34 | TAO D, WANG S P, LIU Y C, et al. Lithium vanadium phosphate as cathode material for lithium ion batteries[J]. Ionics, 2015, 21(5): 1201-1239. |
| 35 | FANG Y J, ZHANG J X, XIAO L F, et al. Phosphate framework electrode materials for sodium ion batteries[J]. Advanced Science, 2017, 4(5): 1600392. |
| 36 | HAN J, LI G N, LIU F, et al. Investigation of K3V2(PO4)3/C nanocomposites as high-potential cathode materials for potassium-ion batteries[J]. Chemical Communications, 2017, 53(11): 1805-1808. |
| 37 | WU X L, JIANG L Y, CAO F F, et al. LiFePO4 nanoparticles embedded in a nanoporous carbon matrix: Superior cathode material for electrochemical energy-storage devices[J]. Advanced Materials, 2009, 21(25/26): 2710-2714. |
| 38 | SUN C W, RAJASEKHARA S, GOODENOUGH J B, et al. Monodisperse porous LiFePO4 microspheres for a high power Li-ion battery cathode[J]. Journal of the American Chemical Society, 2011, 133(7): 2132-2135. |
| 39 | WANG X P, NIU C J, MENG J S, et al. Novel K3V2(PO4)3/C bundled nanowires as superior sodium-ion battery electrode with ultrahigh cycling stability[J]. Advanced Energy Materials, 2015, 5(17): 1500716. |
| 40 | GOKULAKRISHNAN N, PANDURANGAN A, SINHA P K. Catalytic wet peroxide oxidation technique for the removal of decontaminating agents ethylenediaminetetraacetic acid and oxalic acid from aqueous solution using efficient Fenton type Fe-MCM-41 mesoporous materials[J]. Industrial & Engineering Chemistry Research, 2009, 48(3): 1556-1561. |
| 41 | NASRI S, MEGDICHE M, GARGOURI M. Electrical conduction and dielectric properties of a newly synthesized single phase: Ag0.4Na0.6FeP2O7[J]. Physica B: Condensed Matter, 2014, 451: 120-127. |
| 42 | LIU H, STROBRIDGE F C, BORKIEWICZ O, et al. Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes[J]. Science, 2014, 344(6191): 1480. |
| 43 | ZHANG L, ZHANG B W, WANG C R, et al. Constructing the best symmetric full K-ion battery with the NASICON-type K3V2(PO4)3[J]. Nano Energy, 2019, 60: 432-439. |
| 44 | JENKINS T, ALARCO J A, MACKINNON I D R. Synthesis and characterization of a novel hydrated layered vanadium(III) phosphate phase K3V3(PO4)4 ·H2O: A functional cathode material for potassium-ion batteries[J]. ACS Omega, 2021, 6(3): 1917-1929. |
| 45 | SHIRATSUCHI T, OKADA S, YAMAKI J, et al. FePO4 cathode properties for Li and Na secondary cells[J]. Journal of Power Sources, 2006, 159(1): 268-271. |
| 46 | MATHEW V, KIM S, KANG J, et al. Amorphous iron phosphate: Potential host for various charge carrier ions[J]. NPG Asia Materials, 2014, 6(10): e138. |
| 47 | LIM S Y, LEE J H, KIM S, et al. Lattice water for the enhanced performance of amorphous iron phosphate in sodium-ion batteries[J]. ACS Energy Letters, 2017, 2(5): 998-1004. |
| 48 | HOSAKA T, SHIMAMURA T, KUBOTA K, et al. Polyanionic compounds for potassium-ion batteries[J]. The Chemical Record, 2019, 19(4): 735-745. |
| 49 | SULTANA I, RAHMAN M M, MATETI S, et al. Approaching reactive KFePO4 phase for potassium storage by adopting an advanced design strategy[J]. Batteries & Supercaps, 2020, 3(5): 450-455. |
| 50 | YANG S B, CUI G L, PANG S P, et al. Fabrication of cobalt and cobalt oxide/graphene composites: Towards high-performance anode materials for lithium ion batteries[J]. ChemSusChem, 2010, 3(2): 236-239. |
| 51 | WANG D W, LI F, LIU M, et al. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage[J]. Angewandte Chemie International Edition, 2009, 48(9): 1525. |
| 52 | HAN J, NIU Y B, BAO S J, et al. Nanocubic KTi2(PO4)3 electrodes for potassium-ion batteries[J]. Chemical Communications, 2016, 52(78): 11661-11664. |
| 53 | VORONINA N, JO J H, KONAROV A, et al. KTi2(PO4)3 electrode with a long cycling stability for potassium-ion batteries[J]. Small, 2020, 16(20): 2001090. |
| 54 | PARK W B, HAN S C, PARK C, et al. KVP2O7 as a robust high-energy cathode for potassium-ion batteries: Pinpointed by a full screening of the inorganic registry under specific search conditions[J]. Advanced Energy Materials, 2018, 8(13): 1703099. |
| 55 | HE X D, LIAO J Y, CHEN T, et al. Spray drying derived wrinkled pea-shaped carbon-matrixed KVP2O7 as a cathode material for potassium-ion batteries[J]. Journal of Alloys and Compounds, 2021, 884: 161126. |
| 56 | KEE Y, DIMOV N, STAIKOV A, et al. Insight into the limited electrochemical activity of NaVP2O7[J]. RSC Advances, 2015, 5(80): 64991-64996. |
| 57 | WERNERT R, NGUYEN L H B, PETIT E, et al. Controlling the cathodic potential of KVPO4F through oxygen substitution[J]. Chemistry of Materials, 2022, 34(10): 4523-4535. |
| 58 | HE X D, ZHANG L M, JIANG C H, et al. Elevating cyclability of an advanced KVPO4F cathode via multi-component coating strategy for high-performance potassium-ion batteries[J]. Chemical Engineering Journal, 2022, 433: 134634. |
| 59 | WERNERT R, IADECOLA A, STIEVANO L, et al. Origin of vanadium site sequential oxidation in KxVPO4F1– yOy[J]. Chemistry of Materials, 2023, 35(2): 617-627. |
| 60 | PARK H, KIM M, KANG S, et al. Understanding the effects of oxygen defects on the redox reaction pathways in LiVPO4F by combining ab initio calculations with experiments[J]. Journal of Materials Chemistry A, 2019, 7(21): 13060-13070. |
| 61 | LIAO M C, CAO Y J, LI Z Y, et al. VPO4F fluorophosphates polyanion cathodes for high-voltage proton storage[J]. Angewandte Chemie International Edition, 2022, 61(32): e202206635. |
| 62 | CHIHARA K, KATOGI A, KUBOTA K, et al. KVPO4F and KVOPO4 toward 4 volt-class potassium-ion batteries[J]. Chemical Communications, 2017, 53(37): 5208-5211. |
| 63 | ZHAO J, QIN Y Y, LI L, et al. Pillar strategy enhanced ion transport and structural stability toward ultra-stable KVPO4F cathode for practical potassium-ion batteries[J]. Science Bulletin, 2023, 68(6): 593-602. |
| 64 | XIE C, LIU X W, HAN J, et al. Pomegranate-like KVPO4F@C microspheres as high-volumetric-energy-density cathode for potassium-ion batteries[J]. Small, 2022, 18(51): 2204348. |
| 65 | LIN X Y, HUANG J Q, TAN H, et al. K3V2(PO4)2F3 as a robust cathode for potassium-ion batteries[J]. Energy Storage Materials, 2019, 16: 97-101. |
| 66 | MATTS I L, DACEK S, PIETRZAK T K, et al. Explaining performance-limiting mechanisms in fluorophosphate Na-ion battery cathodes through inactive transition-metal mixing and first-principles mobility calculations[J]. Chemistry of Materials, 2015, 27(17): 6008-6015. |
| 67 | FEDOTOV S S, KHASANOVA N R, SAMARIN A S, et al. AVPO4F (A=Li, K): A 4 V cathode material for high-power rechargeable batteries[J]. Chemistry of Materials, 2016, 28(2): 411-415. |
| 68 | HE H Y, CAO K, GUO S, et al. Flower-like K1+ δVOPO4F crystallite with a layered framework structure as a robust cathode for potassium-ion batteries[J]. Journal of Power Sources, 2023, 564: 232862. |
| 69 | LIU Z M, WANG J, LU B G. Plum pudding model inspired KVPO4F@3DC as high-voltage and hyperstable cathode for potassium ion batteries[J]. Science Bulletin, 2020, 65(15): 1242-1251. |
| 70 | RECHAM N, ROUSSE G, SOUGRATI M T, et al. Preparation and characterization of a stable FeSO4F-based framework for alkali ion insertion electrodes[J]. Chemistry of Materials, 2012, 24(22): 4363-4370. |
| 71 | LANDER L, ROUSSE G, ABAKUMOV A M, et al. Structural, electrochemical and magnetic properties of a novel KFeSO4F polymorph[J]. Journal of Materials Chemistry A, 2015, 3(39): 19754-19764. |
| 72 | LING C, MIZUNO F. Mechanistic study of the electrochemical extraction of K+ from KFeSO4F[J]. Journal of Materials Chemistry A, 2013, 1(27): 8000-8006. |
| 73 | KUMAR P R, HOSAKA T, SHIMAMURA T, et al. Mg-doped KFeSO4F as a high-performance cathode material for potassium-ion batteries[J]. ACS Applied Energy Materials, 2022, 5(11): 13470-13479. |
| 74 | LIAO J Y, HU Q, DU Y C, et al. Robust carbon nanotube-interwoven KFeSO4F microspheres as reliable potassium cathodes[J]. Science Bulletin, 2022, 67(21): 2208-2215. |
| 75 | DONG J M, LIAO J Y, HE X D, et al. Graphene encircled KFeSO4F cathode composite for high energy density potassium-ion batteries[J]. Chemical Communications, 2020, 56(69): 10050-10053. |
| 76 | LIAO J Y, ZHANG X X, ZHANG Q H, et al. Synthesis of KVPO4F/carbon porous single crystalline nanoplates for high-rate potassium-ion batteries[J]. Nano Letters, 2022, 22(12): 4933-4940. |
| 77 | GU Z Y, GUO J Z, SUN Z H, et al. Air/water/temperature-stable cathode for all-climate sodium-ion batteries[J]. Cell Reports Physical Science, 2021, 2(12): 100665. |
| 78 | LIU Y, SUN C, NI Q, et al. Enhanced electrochemical performance of NASICON-type sodium ion cathode based on charge balance theory[J]. Energy Storage Materials, 2022, 53: 881-889. |
| 79 | 赵易飞, 杨振东, 李凤, 等. 氮掺杂碳包覆Na3V2(PO4)2F3钠离子电池正极材料的制备与性能[J]. 储能科学与技术, 2022, 11(6)1883-1891 |
| ZHAO Y F, YANG Z D, LI F, et al. Nitrogen-doped carbon-coated Na3V2(PO4)2F3 cathode materials for sodium-ion batteries: Preparation and electrochemical performance[J]. Energy Storage Science and Technology, 2022, 11(6)1883-1891 | |
| 80 | FEDOTOV S S, SAMARIN A S, NIKITINA V A, et al. Reversible facile Rb+ and K+ ions de/insertion in a KTiOPO4-type RbVPO4F cathode material[J]. Journal of Materials Chemistry A, 2018, 6(29): 14420-14430. |
| [1] | 赵玉文, 杨欢, 郭俊朋, 张毅, 孙琦, 张志佳. 磁性金属元素在钠离子电池中的应用[J]. 储能科学与技术, 2023, 12(5): 1332-1347. |
| [2] | 王轩臣, 王达, 刘朝孟, 高宣雯, 骆文彬. 钾离子电池电解液的研究进展及展望[J]. 储能科学与技术, 2023, 12(5): 1409-1426. |
| [3] | 韩文哲, 赖青松, 高宣雯, 骆文彬. 钾离子电池锰基层状氧化物正极的研究进展[J]. 储能科学与技术, 2023, 12(5): 1364-1379. |
| [4] | 朱璟, 申晓宇, 岑官骏, 乔荣涵, 郝峻丰, 季洪祥, 田孟羽, 金周, 詹元杰, 武怿达, 闫勇, 贲留斌, 俞海龙, 刘燕燕, 黄学杰. 锂电池百篇论文点评(2023.2.1—2023.3.31)[J]. 储能科学与技术, 2023, 12(5): 1553-1569. |
| [5] | 刘畅, 姚君君, 孙颖, 冯大明, 郑宏杰, 马天翼. 乳化/氧化协同制备高倍率钾离子电池负极用沥青基碳微球[J]. 储能科学与技术, 2023, 12(5): 1444-1452. |
| [6] | 李金涛, 牟粤, 王静, 邱景义, 明海. 高镍正极材料的稳定改性方法研究综述[J]. 储能科学与技术, 2023, 12(5): 1636-1654. |
| [7] | 申晓宇, 朱璟, 岑官骏, 乔荣涵, 郝峻丰, 田孟羽, 季洪祥, 金周, 武怿达, 詹元杰, 闫勇, 贲留斌, 俞海龙, 刘燕燕, 黄学杰. 锂电池百篇论文点评(2022.12.1—2023.1.31)[J]. 储能科学与技术, 2023, 12(3): 639-653. |
| [8] | 张德柳, 张言, 王海, 王佳东, 高宣雯, 刘朝孟, 杨东润, 骆文彬. 镁掺杂协同氧化铝包覆优化锂离子电池高镍正极材料[J]. 储能科学与技术, 2023, 12(2): 339-348. |
| [9] | 田孟羽, 武怿达, 郝峻丰, 朱璟, 岑官骏, 乔荣涵, 申晓宇, 季洪祥, 金周, 詹元杰, 闫勇, 贲留斌, 俞海龙, 刘燕燕, 黄学杰. 锂电池百篇论文点评(2022.10.01—2022.11.30)[J]. 储能科学与技术, 2023, 12(1): 1-15. |
| [10] | 张凯, 徐友龙. 钠离子电池锰酸钠正极材料研究进展与发展趋势[J]. 储能科学与技术, 2023, 12(1): 86-110. |
| [11] | 俎梦杨, 张梦, 李子坤, 黄令. 高镍NCA、NCM及NCMA材料循环容量衰减机理研究[J]. 储能科学与技术, 2023, 12(1): 51-60. |
| [12] | 王绍聪, 李伟, 黄瑞琴, 郭艺飞, 刘峥. 锰基钠离子电池正极材料Jahn-Teller效应抑制方法进展[J]. 储能科学与技术, 2023, 12(1): 139-149. |
| [13] | 朱璟, 武怿达, 郝峻丰, 岑官骏, 乔荣涵, 申晓宇, 田孟羽, 季洪祥, 金周, 詹元杰, 闫勇, 贲留斌, 俞海龙, 刘燕燕, 黄学杰. 锂电池百篇论文点评(2022.6.1—2022.7.31)[J]. 储能科学与技术, 2022, 11(9): 3035-3050. |
| [14] | 郭凯强, 车海英, 张浩然, 廖建平, 周煌, 张云龙, 陈航达, 申展, 刘海梅, 马紫峰. B2O3 包覆NaNi1/3Fe1/3Mn1/3O2 正极材料制备及其电化学性能[J]. 储能科学与技术, 2022, 11(9): 2980-2988. |
| [15] | 栗志展, 秦金磊, 梁嘉宁, 李峥嵘, 王瑞, 王得丽. 高镍三元层状锂离子电池正极材料:研究进展、挑战及改善策略[J]. 储能科学与技术, 2022, 11(9): 2900-2920. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
