Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (1): 157-166.doi: 10.19799/j.cnki.2095-4239.2023.0816
Previous Articles Next Articles
Yimei OUYANG( ), Mengmeng ZHAO, Guiming ZHONG(
), Mengmeng ZHAO, Guiming ZHONG( ), Zhangquan PENG(
), Zhangquan PENG( )
)
Received:2023-11-14
Revised:2023-12-02
Online:2024-01-05
Published:2024-01-22
Contact:
Guiming ZHONG, Zhangquan PENG
E-mail:ouyangyimei@dicp.ac.cn;gmzhong@dicp.ac.cn;zqpeng@dicp.ac.cn
CLC Number:
Yimei OUYANG, Mengmeng ZHAO, Guiming ZHONG, Zhangquan PENG. Nuclear magnetic resonance spectroscopy for probing interfaces in electrochemical energy storage systems[J]. Energy Storage Science and Technology, 2024, 13(1): 157-166.
Table 1
Commonly used nuclear information for NMR studies of electrode-electrolyte interphase layers"
| 核磁同位素 | 自旋量子数 | 天然 丰度/% | 四极矩Q/fm2 | 灵敏度 | 应用 | 识别界面物种 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1H | 1/2 | 99.98 | — | 高 | 化学位移识别界面有机物、含氢无机物 | LiOH、LiH、HCO2Li、CH3OLi、CH3OCO2Li等 | [ |
| 7Li | 3/2 | 92.41 | -4.01 | 高 | 化学位移和四极耦合用于识别无机相 | LiF、Li2CO3、LiOH、LiH、Li2O等 | [ |
| 13C | 1/2 | 1.07 | — | 低 | 化学位移识别界面无机相(辅助同位素富集、交叉极化、动态核极化技术提高灵敏度) | CH3OLi、HCO2Li、CH3R、R'CH2R、 CH3OCO2Li、Li2CO3等 | [ |
| 19F | 1/2 | 100 | — | 高 | 化学位移识别界面含氟无机物、有机物 | LiF、RPO3F、RPO2F2等 | [ |
| 23Na | 3/2 | 100 | 10.4 | 高 | 化学位移和四极耦合用于识别无机相 | NaF、Na2CO3、NaOH、NaH、Na2O等 | [ |
| 31P | 1/2 | 100 | — | 高 | 化学位移识别界面含磷无机相 | RPO3F、RPO2F2等 | [ |

Fig. 4
(a) Schematic diagram of the migration path of lithium ions in the composite solid electrolyte[35]; (b) The affect of space charge layer on the interface of LiV205-LAGP and Li2V2O5-LAGP[37]; (c) Lithium ion transport mechanism of composite electrolyth[38]; (d) Schematic diagram of 6Li symmetrical battery and lithlum ion transport pathway after cycling[39]"
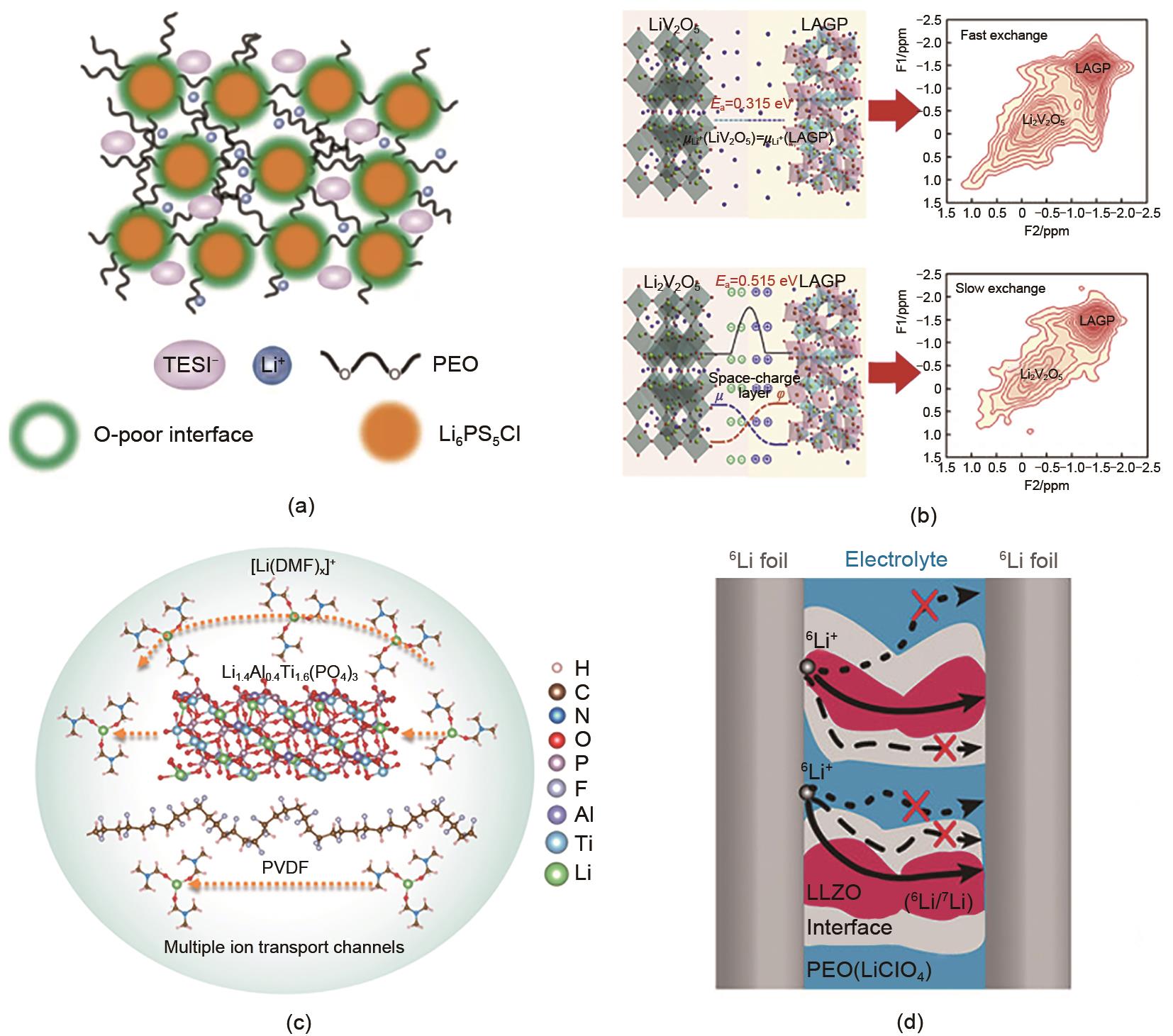
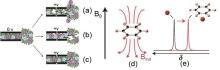
Fig. 5
Schematic diagram of possible charge storage mechanisms when the electrode surface is positively charged[41]: (a) Common ion rejection; (b) Counterion adsorption; (c) Counterion and common ion exchange; (d) Schematic diagram of the ring current effect principle; (e) Compared with the free solution, the chemical shift of nuclear substances near the ring system is negatively shifted (red)[45-46]"

Fig. 6
In-situ19F NMR dynamically monitors the change of anion intensity in the porous carbon electrode[48]: (a) positive electrode; (b) negative electrode; in-situ31P and 19F NMR dynamically monitors the adsorption of cations and anions in the porous carbon electrode[51]: (c) positive electrode; (d) negative electrode"

| 1 | 邓诗维, 吴剑芳, 时拓. 固体电解质缺陷化学分析:晶粒体点缺陷及晶界空间电荷层[J]. 储能科学与技术, 2022, 11(3): 939-947. |
| DENG S W, WU J F, SHI T. Defect chemistry analysis of solid electrolytes: Point defects in grain bulk and grain boundary space-charge layer[J]. Energy Storage Science and Technology, 2022, 11(3): 939-947. | |
| 2 | CHAPMAN D L. LI. A contribution to the theory of electrocapillarity[J]. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 1913, 25(148): 475-481. |
| 3 | GRAHAME D C. The electrical double layer and the theory of electrocapillarity[J]. Chemical Reviews, 1947, 41(3): 441-501. |
| 4 | PELED E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model[J]. Journal of the Electrochemical Society, 1979, 126(12): 2047-2051. |
| 5 | KRANZ S, KRANZ T, JAEGERMANN A G, et al. Is the solid electrolyte interphase in lithium-ion batteries really a solid electrolyte? Transport experiments on lithium bis(oxalato)borate-based model interphases[J]. Journal of Power Sources, 2019, 418: 138-146. |
| 6 | XU C, SUN B, GUSTAFSSON T, et al. Interface layer formation in solid polymer electrolyte lithium batteries: An XPS study[J]. Journal of Materials Chemistry A, 2014, 2(20): 7256-7264. |
| 7 | LIU B W, YU X Y, ZHU Z H, et al. In situ chemical probing of the electrode-electrolyte interface by ToF-SIMS[J]. Lab on a Chip, 2014, 14(5): 855-859. |
| 8 | YAN C, CHENG X B, TIAN Y, et al. Lithium metal anodes: Dual-layered film protected lithium metal anode to enable dendrite-free lithium deposition[J]. Advanced Materials, 2018, 30(25): doi:10.1002/adma.201707629. |
| 9 | NIE M Y, CHALASANI D, ABRAHAM D P, et al. Lithium ion battery graphite solid electrolyte interphase revealed by microscopy and spectroscopy[J]. The Journal of Physical Chemistry C, 2013, 117(3): 1257-1267. |
| 10 | HABER S, LESKES M. What can we learn from solid state NMR on the electrode-electrolyte interface?[J]. Advanced Materials, 2018, 30(41): doi: 10.1002/adma.201706496. |
| 11 | DUPRE N, CUISINIER M, GUYOMARD D. Electrode/electrolyte interface studies in lithium batteries using NMR[J]. Interface Magazine, 2011, 20(3): 61-67. |
| 12 | DUER M J. Solid state NMR spectroscopy: principles and applications[M]. John Wiley & Sons, 2008. |
| 13 | O'DELL L A. The WURST kind of pulses in solid-state NMR[J]. Solid State Nuclear Magnetic Resonance, 2013, 55(56): 28-41. |
| 14 | AURBACH D, GAMOLSKY K, MARKOVSKY B, et al. On the use of vinylene carbonate (VC) as an additive to electrolyte solutions for Li-ion batteries[J]. Electrochimica Acta, 2002, 47(9): 1423-1439. |
| 15 | ZHANG X E, WANG S, XUE C J, et al. Self-suppression of lithium dendrite in all-solid-state lithium metal batteries with poly (vinylidene difluoride)-based solid electrolytes[J]. Advanced Materials, 2019, 31(11): doi: 10.1002/adma.1806082. |
| 16 | ZHANG Z Y, SMITH K, JERVIS R, et al. Operando electrochemical atomic force microscopy of solid-electrolyte interphase formation on graphite anodes: The evolution of SEI morphology and mechanical properties[J]. ACS Applied Materials & Interfaces, 2020, 12(31): 35132-35141. |
| 17 | LI Y Z, LI Y B, PEI A, et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy[J]. Science, 2017, 358(6362): 506-510. |
| 18 | LI Q, LIU X S, HAN X A, et al. Identification of the solid electrolyte interface on the Si/C composite anode with FEC as the additive[J]. ACS Applied Materials & Interfaces, 2019, 11(15): 14066-14075. |
| 19 | HU Y Y, LIU Z G, NAM K W, et al. Origin of additional capacities in metal oxide lithium-ion battery electrodes[J]. Nature Materials, 2013, 12(12): 1130-1136. |
| 20 | WAN C A, XU S C, HU M Y, et al. Multinuclear NMR study of the solid electrolyte interface formed in lithium metal batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(17): 14741-14748. |
| 21 | MICHAN A L, LESKES M, GREY C P. Voltage dependent solid electrolyte interphase formation in silicon electrodes: Monitoring the formation of organic decomposition products[J]. Chemistry of Materials, 2016, 28(1): 385-398. |
| 22 | XIANG Y X, ZHENG G R, LIANG Z T, et al. Visualizing the growth process of sodium microstructures in sodium batteries by in situ 23Na MRI and NMR spectroscopy[J]. Nature Nanotechnology, 2020, 15(10): 883-890. |
| 23 | MAY R, FRITZSCHING K J, LIVITZ D, et al. Rapid interfacial exchange of Li ions dictates high coulombic efficiency in Li metal anodes[J]. ACS Energy Letters, 2021: 1162-1169. |
| 24 | MEYER B M, LEIFER N, SAKAMOTO S, et al. High field multinuclear NMR investigation of the SEI layer in lithium rechargeable batteries[J]. Electrochemical and Solid-State Letters, 2005, 8(3): A145. |
| 25 | REEVE Z E M, FRANKO C J, HARRIS K J, et al. Detection of electrochemical reaction products from the sodium-oxygen cell with solid-state 23Na NMR spectroscopy[J]. Journal of the American Chemical Society, 2017, 139(2): 595-598. |
| 26 | GAO L N, CHEN J E, CHEN Q L, et al. The chemical evolution of solid electrolyte interface in sodium metal batteries[J]. Science Advances, 2022, 8(6): eabm4606. |
| 27 | ZHENG G R, XIANG Y X, CHEN S J, et al. Additives synergy for stable interface formation on rechargeable lithium metal anodes[J]. Energy Storage Materials, 2020, 29: 377-385. |
| 28 | HABER S, LESKES M. Dynamic Nuclear Polarization in battery materials[J]. Solid State Nuclear Magnetic Resonance, 2022, 117: 101763. |
| 29 | LESKES M, KIM G, LIU T, et al. Surface-sensitive NMR detection of the solid electrolyte interphase layer on reduced graphene oxide[J]. The Journal of Physical Chemistry Letters, 2017, 8(5): 1078-1085. |
| 30 | JIN Y T, KNEUSELS N J H, MAGUSIN P C M M, et al. Identifying the structural basis for the increased stability of the solid electrolyte interphase formed on silicon with the additive fluoroethylene carbonate[J]. Journal of the American Chemical Society, 2017, 139(42): 14992-15004. |
| 31 | JIN Y T, KNEUSELS N J H, MARBELLA L E, et al. Understanding fluoroethylene carbonate and vinylene carbonate based electrolytes for Si anodes in lithium ion batteries with NMR spectroscopy[J]. Journal of the American Chemical Society, 2018, 140(31): 9854-9867. |
| 32 | HOPE M A, RINKEL B L D, GUNNARSDÓTTIR A B, et al. Selective NMR observation of the SEI-metal interface by dynamic nuclear polarisation from lithium metal[J]. Nature Communications, 2020, 11: 2224. |
| 33 | HUNG I, ZHOU L N, POURPOINT F, et al. Isotropic high field NMR spectra of Li-ion battery materials with anisotropy >1 MHz[J]. Journal of the American Chemical Society, 2012, 134(4): 1898-1901. |
| 34 | COLUMBUS D, ARUNACHALAM V, GLANG F, et al. Direct detection of lithium exchange across the solid electrolyte interphase by 7Li chemical exchange saturation transfer[J]. Journal of the American Chemical Society, 2022, 144(22): 9836-9844. |
| 35 | LIU M, ZHANG S N, VAN ECK E R H, et al. Improving Li-ion interfacial transport in hybrid solid electrolytes[J]. Nature Nanotechnology, 2022, 17(9): 959-967. |
| 36 | 陈骋, 凌仕刚, 郭向欣, 等. 固态锂二次电池关键材料中的空间电荷层效应: 原理和展望[J]. 储能科学与技术, 2016, 5(5): 668-677. |
| CHEN C, LING S G, GUO X X, et al. Space charge layer effect in rechargeable solid state lithium batteries: Principle and perspective[J]. Energy Storage Science and Technology, 2016, 5(5): 668-677. | |
| 37 | CHENG Z, LIU M, GANAPATHY S, et al. Revealing the impact of space-charge layers on the Li-ion transport in all-solid-state batteries[J]. Joule, 2020, 4(6): 1311-1323. |
| 38 | YANG K, CHEN L K, MA J B, et al. Stable interface chemistry and multiple ion transport of composite electrolyte contribute to ultra-long cycling solid-state LiNi0.8Co0.1Mn0.1O2/lithium metal batteries[J]. Angewandte Chemie International Edition, 2021, 60(46): 24668-24675. |
| 39 | ZHENG J, TANG M X, HU Y Y. Lithium ion pathway within Li7La3Zr2O12-polyethylene oxide composite electrolytes[J]. Angewandte Chemie International Edition, 2016, 55(40): 12538-12542. |
| 40 | PELED E, MENKIN S. Review—SEI: Past, present and future[J]. Journal of the Electrochemical Society, 2017, 164(7): A1703-A1719. |
| 41 | GRIFFIN J M, FORSE A C, WANG H, et al. Ion counting in supercapacitor electrodes using NMR spectroscopy[J]. Faraday Discussions, 2014, 176(0): 49-68. |
| 42 | LEVI M D, SALITRA G, LEVY N, et al. Application of a quartz-crystal microbalance to measure ionic fluxes in microporous carbons for energy storage[J]. Nature Materials, 2009, 8(11): 872-875. |
| 43 | BOUKHALFA S, HE L, MELNICHENKO Y B, et al. Small-angle neutron scattering for in situ probing of ion adsorption inside micropores[J]. Angewandte Chemie International Edition, 2013, 52(17): 4618-4622. |
| 44 | SHARMA K, BILHEUX H Z, WALKER L M H, et al. Neutron imaging of ion transport in mesoporous carbon materials[J]. Physical Chemistry Chemical Physics, 2013, 15(28): 11740-11747. |
| 45 | LAZZERETTI P. Ring Currents[J]. Progress in Nuclear Magnetic Resonance Spectroscopy, 2000, 36(1): 1-88. |
| 46 | YANG Y, FU R, HUO H. NMR and MRI of electrochemical energy storage materials and devices[M]. Royal Society of Chemistry, 2021. |
| 47 | WANG H, KÖSTER T K J, TREASE N M, et al. Real-time NMR studies of electrochemical double-layer capacitors[J]. Journal of the American Chemical Society, 2011, 133(48): 19270-19273. |
| 48 | WANG H, FORSE A C, GRIFFIN J M, et al. In situ NMR spectroscopy of supercapacitors: Insight into the charge storage mechanism[J]. Journal of the American Chemical Society, 2013, 135(50): 18968-18980. |
| 49 | GRIFFIN J M, FORSE A C, TSAI W Y, et al. In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors[J]. Nature Materials, 2015, 14(8): 812-819. |
| 50 | FORSE A C, GRIFFIN J M, GREY C P. Selective observation of charge storing ions in supercapacitor electrode materials[J]. Solid State Nuclear Magnetic Resonance, 2018, 89: 45-49. |
| 51 | SU X L, YE J L, ZHU Y W. Advances in in-situ characterizations of electrode materials for better supercapacitors[J]. Journal of Energy Chemistry, 2021, 54: 242-253. |
| [1] | Chu ZHANG, Dongcai CHEN, Xiangping CHEN, Yongxiang CAI. Economic benefit analysis of optimal allocation of energy storage in multiple application scenarios [J]. Energy Storage Science and Technology, 2024, 13(6): 2078-2088. |
| [2] | Kun ZENG, Xiaoyan ZHENG, Huiling GONG, Bo ZOU, Kai CHEN, Zhongna YAN. Research progress in liquid metal batteries based on lithium negative electrodes [J]. Energy Storage Science and Technology, 2024, 13(1): 299-310. |
| [3] | Su YAN, Fangfang ZHONG, Junwei LIU, Mei DING, Chuankun JIA. Key materials and advanced characterization of high-energy-density flow battery [J]. Energy Storage Science and Technology, 2024, 13(1): 143-156. |
| [4] | Libo ZHANG, Gege WANG. Topic identification, evolution, and risk analysis of electrochemical energy storage battery technology [J]. Energy Storage Science and Technology, 2023, 12(8): 2680-2692. |
| [5] | Guangjin ZHAO, Bowen LI, Yuxia HU, Ruifeng DONG, Fangfang WANG. Overview of the echelon utilization technology and engineering application of retired power batteries [J]. Energy Storage Science and Technology, 2023, 12(7): 2319-2332. |
| [6] | Zhixiang CHENG, Wei CAO, Bo HU, Yunfang CHENG, Xin LI, Lihua JIANG, Kaiqiang JIN, Qingsong WANG. Thermal runaway and explosion propagation characteristics of large lithium iron phosphate battery for energy storage station [J]. Energy Storage Science and Technology, 2023, 12(3): 923-933. |
| [7] | Xuanliang ZHANG, Ting HE, Wenlong ZHU, Shen WANG, Jianhua ZENG, Quan XU, Yingchun NIU. A SOH estimation model for energy storage batteries based on multiple cycle features [J]. Energy Storage Science and Technology, 2023, 12(11): 3488-3498. |
| [8] | Yang LIU, Weijun TENG, Qingfa GU, Xin SUN, Yuliang TAN, Zhijin FANG, Jianlin LI. Scaled-up diversified electrochemical energy storage LCOE and its economic analysis [J]. Energy Storage Science and Technology, 2023, 12(1): 312-318. |
| [9] | Hong LI, Qiang ZHANG. A review of energy storage science and technology projects supported by national key R&D program [J]. Energy Storage Science and Technology, 2022, 11(9): 2691-2701. |
| [10] | Zhicheng CAO, Kaiyun ZHOU, Jiali ZHU, Gaoming LIU, Min YAN, Shun TANG, Yuancheng CAO, Shijie CHENG, Weixin ZHANG. Patent analysis of fire-protection technology of lithium-ion energy storage system [J]. Energy Storage Science and Technology, 2022, 11(8): 2664-2670. |
| [11] | Nan LIN, Ulrike KREWER, Jochen ZAUSCH, Konrad STEINER, Haibo LIN, Shouhua FENG. Development and application of multiphysics models for electrochemical energy storage and conversion systems [J]. Energy Storage Science and Technology, 2022, 11(4): 1149-1164. |
| [12] | Siqi SHI, Zhangwei TU, Xinxin ZOU, Shiyu SUN, Zhengwei YANG, Yue LIU. Applying data-driven machine learning to studying electrochemical energy storage materials [J]. Energy Storage Science and Technology, 2022, 11(3): 739-759. |
| [13] | Zhiwei ZHAO, Zhi YANG, Zhangquan PENG. Application of time-of-flight secondary ion mass spectrometry in lithium-based rechargeable batteries [J]. Energy Storage Science and Technology, 2022, 11(3): 781-794. |
| [14] | Mengyao QI, Yichen HOU, Lei CHEN, Lijun YANG. Numerical simulation of a novel radial all-vanadium flow battery cell [J]. Energy Storage Science and Technology, 2022, 11(10): 3209-3220. |
| [15] | Yun TANG, Fang YUE, Kaimo GUO, Lanchun LI, Wei CHEN. International development trend analysis of next-generation electrochemical energy storage technology [J]. Energy Storage Science and Technology, 2022, 11(1): 89-97. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
