Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (5): 1636-1654.doi: 10.19799/j.cnki.2095-4239.2023.0052
• Energy Storage Materials and Devices • Previous Articles Next Articles
Jintao LI1( ), Yue MU2,3, Jing WANG1(
), Yue MU2,3, Jing WANG1( ), Jingyi QIU3, Hai MING3(
), Jingyi QIU3, Hai MING3( )
)
Received:2023-02-06
Revised:2023-03-01
Online:2023-05-05
Published:2023-05-29
Contact:
Jing WANG, Hai MING
E-mail:leejt99@163.com;jwang6027@ysu.edu.cn;hai.mingenergy@ hotmail.com
CLC Number:
Jintao LI, Yue MU, Jing WANG, Jingyi QIU, Hai MING. Investigation of the structural evolution and interface behavior in cathode materials for Li-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(5): 1636-1654.

Fig. 3
SEM images of bare LiNi0.8Co0.1Mn0.1O2 and 2% PDMS solution-processed LiNi0.8Co0.1Mn0.1O2 for different exposure times: (a) fresh, (b) 24 h exposed, (c) 72 h exposed, and (d) 120 h exposed bare LiNi0.8Co0.1Mn0.1O2; (e) fresh, (f) 24 h exposed, (g) 72 h exposed, and (h) 120 h exposed SiMH-LiNi0.8Co0.1Mn0.1O2[38]"
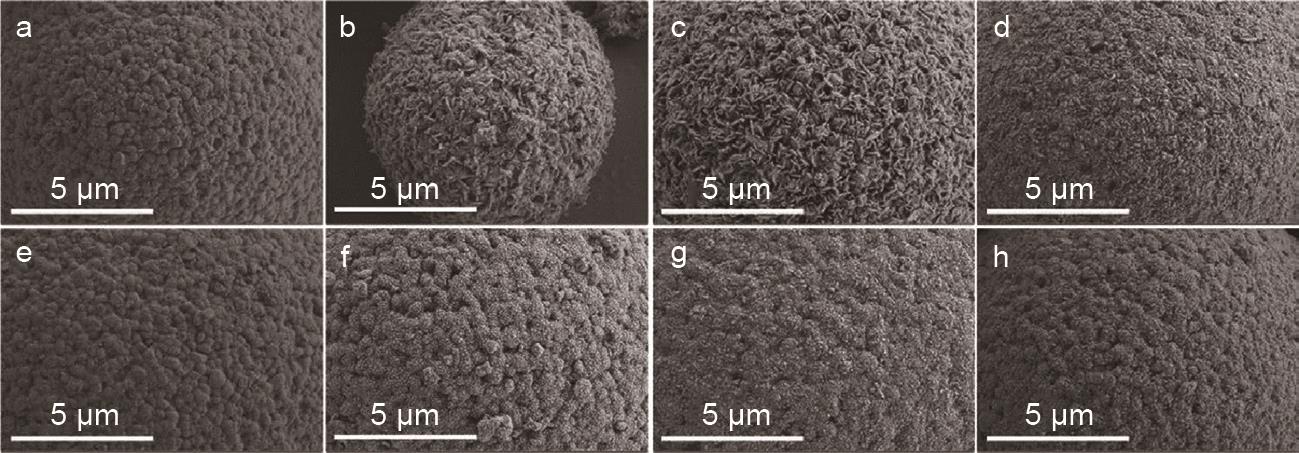
Table 1
Application of concentration gradient design in recent years"
| 梯度策略 | 制备方法 | 应用电池类型 | 主要性能 | 文献 |
|---|---|---|---|---|
| 全浓度梯度 | 两股进料共沉淀法 | 扣式电池 | 保持率90%@100th(50 ℃ 5 C) | 2019[ |
| 梯度外壳 | 差分共沉淀法 | 扣式、袋式电池 | 放电容量229 mAh/g保持率88%@1000th(1 C) | 2019[ |
| Ni/Mn和Al双浓度梯度 | 溶剂热法控制 | 扣式电池 | 保持率84.1%@400th(1.5 C) 电压衰减0.97 mV/圈(0.5 C) | 2021[ |
两级粒子 双浓度梯度 | 共沉淀 煅烧混合法 | 扣式、袋式电池 | 保持率84.1%@500th(1 C) | 2022[ |
Table 2
Several different ion doping applications in recent years"
| 掺杂离子 | 半径/Å | 电压/V | 电流密度/C | 比容量/(mAh/g) | 电流密度/C | 循环性能 | 文献 | ||
|---|---|---|---|---|---|---|---|---|---|
| 掺杂后 | 掺杂前 | ||||||||
| 掺杂后 | 掺杂前 | ||||||||
| Mg2+ | 0.72 | 4.5 | 1 | 199.7 | 201.8 | 1 | 87.2%@200th | 74%@200th | 2020[ |
| Ga3+ | 0.76 | 4.3 | 0.1 | 246.8 | 233.2 | 0.5 | 90.1%@100th | 68.5%@100th | 2022[ |
| Zr4+ | 0.72 | 4.4 | 0.1 | 225.2 | 230.1 | 0.3 | 83%@100th | 68%@100th | 2021[ |
| F- | 1.33 | — | — | — | — | 0.5 | 96.8%@100th | 60.7%@100th | 2021[ |
| S2- | 1.84 | 4.5 | 0.05 | 270.5 | 261.3 | 0.5 | 81.10%@600th | 65.78%@200th | 2019[ |
Table 3
Application of several different coating materials in recent years"
| 类型 | 包覆材料 | 电流密度/C | 电压/V | 容量/(mAh/g) | 循环性能 | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|
| 包覆后 | 包覆前 | 包覆后 | 包覆前 | ||||||
| 盐类 | LiPON | 0.5 | 4.2 | 174.9 | 176.7 | 97.5%@100th | 94.3%@100th | 2022[ | |
| 盐类 | LiTaO3 | 0.1 | 4.3 | 207.9 | 207.4 | 80.3%@150th 60 ℃ | <50%@70th 60 ℃ | 2022[ | |
| 聚合物 | PEDOT | 0.1 | 4.3 | 202 | 198 | 88.7%@100th | 66.3%@100th | 2019[ | |
| 聚合物 | PEG+PANI | 0.1 | 4.5 | 158 | 153 | 88%@100th | 59%@100th | 2022[ | |
| 氧化物 | TiO2 | 1 | 4.4 | 168.7 | 177.2 | 96%@200th | 78%@200th | 2020[ | |
| 氧化物 | V2O5 | 0.2 | 4.3 | 210.4 | 196.6 | 83.4%@100th | 71.4%@100th | 2022[ | |
Table 4
Application of multiple modification methods in recent years"
| 改性策略 | 改性方法 | 主要特点 | 参考文献 |
|---|---|---|---|
Ti4+掺杂 全浓度梯度 Li2ZrO3包覆 | 共沉淀+不同温度煅烧+湿化学法 | 具有优异热稳定性和耐高压性能 | 2023[ |
单晶结构 Nb2+梯度掺杂 | 喷气磨机粉碎+煅烧 | 高温和高电压下均具有优异稳定性和放电比容量 | 2022[ |
浓度梯度 Ti4+掺杂 | 共沉淀+煅烧+球磨 | 具有高循环稳定性 | 2020[ |
组分优化 La2O3包覆 | 共沉淀+研磨+煅烧 | 具有优异的循环稳定性和放电比容量 | 2022[ |
Li2MoO4包覆 Mo6+掺杂 | 研磨+烧结 | 电极耐用性提升,具有高循环稳定性 | 2022[ |
| 1 | LI M, LU J, CHEN Z W, et al. 30 years of lithium-ion batteries[J]. Advanced Materials (Deerfield Beach, Fla), 2018: doi: 10.1002/adma.201800561. |
| 2 | CHANG L, WEI A, LUO S, et al. Lithium‐ion battery: A comprehensive research progress of high nickel ternary cathode material[J]. International Journal of Energy Research, 2022, 46(15): 23145-23172. |
| 3 | GOODENOUGH J B. Evolution of strategies for modern rechargeable batteries[J]. Accounts of Chemical Research, 2013, 46(5): 1053-1061. |
| 4 | LI J Y, LIN C, WENG M Y, et al. Structural origin of the high-voltage instability of lithium cobalt oxide[J]. Nature Nanotechnology, 2021, 16(5): 599-605. |
| 5 | MANTHIRAM A, GOODENOUGH J B. Layered lithium cobalt oxide cathodes[J]. Nature Energy, 2021, 6(3): 323. |
| 6 | MALIK M, CHAN K H, AZIMI G. Review on the synthesis of LiNixMnyCo1- x- yO2 (NMC) cathodes for lithium-ion batteries[J]. Materials Today Energy, 2022, 28: 101066. |
| 7 | JIANG M, DANILOV D L, EICHEL R A, et al. A review of degradation mechanisms and recent achievements for Ni‐rich cathode‐based Li‐ion batteries[J]. Advanced Energy Materials, 2021, 11 (48): 10.1002/aenm.202103005. |
| 8 | ZHENG J X, YE Y K, LIU T C, et al. Ni/Li disordering in layered transition metal oxide: Electrochemical impact, origin, and control[J]. Accounts of Chemical Research, 2019, 52(8): 2201-2209. |
| 9 | YOUNGJIN K, HYOJU P, WARNER JAMIE H, et al. Unraveling the intricacies of residual lithium in high-Ni cathodes for lithium-ion batteries[J]. Acs Energy Letters, 2021, 6(3): 941-948. |
| 10 | LIANG L, ZHANG W, ZHAO F, et al. Surface/interface structure degradation of Ni‐rich layered oxide cathodes toward lithium‐ion batteries: Fundamental mechanisms and remedying strategies[J]. Advanced Materials Interfaces, 2019, 7(3): https://doi.org/10.1002/admi.201901749. |
| 11 | LI H H, YABUUCHI N, MENG Y S, et al. Changes in the cation ordering of layered O3 LixNi0.5Mn0.5O2 during electrochemical cycling to high voltages: An electron diffraction study[J]. Chemistry of Materials, 2007, 19 (10): 2551-2565. |
| 12 | LIN F, MARKUS I M, NORDLUND D, et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries[J]. Nature Communications, 2014, 5: 3529. |
| 13 | KANG K, CEDER G. Factors that affect Li mobility in layered lithium transition metal oxides[J]. Physics Review B, 2006, doi:10.1103/physrevb.74.094105. |
| 14 | LEE J, URBAN A, LI X, et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries[J]. Science, 2014, 343(6170): 519-522. |
| 15 | CHENG C X, TAN L, LIU H W, et al. High rate performances of the cathode material LiNi1/3Co1/3Mn1/3O2 synthesized using low temperature hydroxide precipitation[J]. Materials Research Bulletin, 2011, 46(11): 2032-2035. |
| 16 | KONDRAKOV A O, SCHMIDT A, XU J, et al. Anisotropic lattice strain and mechanical degradation of high- and low-nickel NCM cathode materials for Li-ion batteries[J]. The Journal of Physical Chemistry C, 2017, 121(6): 3286-3294. |
| 17 | KOYAMA Y, ARAI H, TANAKA I, et al. High temperature defect chemistry in layered lithium transition-metal oxides based on first-principles calculations[J]. Journal of Power Sources, 2013, 244: 592-596. |
| 18 | BI Y J, YANG W C, DU R, et al. Correlation of oxygen non-stoichiometry to the instabilities and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 utilized in lithium ion battery[J]. Journal of Power Sources, 2015, 283: 211-218. |
| 19 | HUANG B, CHENG L, LI X Z, et al. Layered cathode materials: Precursors, synthesis, microstructure, electrochemical properties, and battery performance[J]. Small, 2022, 18(20): 2107697. |
| 20 | WANG L F, LIU G Y, DING X N, et al. Simultaneous coating and doping of a nickel-rich cathode by an oxygen ion conductor for enhanced stability and power of lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(37): 33901-33912. |
| 21 | TANG Z F, WANG S, LIAO J Y, et al. Facilitating lithium-ion diffusion in layered cathode materials by introducing Li+/Ni2+ antisite defects for high-rate Li-ion batteries[J]. Research (Washington, D C), 2019, 2019: 2198906. |
| 22 | YOON CHONG S, JAE C M, DO WOOK J, et al. Cation ordering of Zr-doped LiNiO2 cathode for lithium-ion batteries[J]. Chemistry of Materials, 2018, 30(5): 1808-1814. |
| 23 | LI X, ZHANG K J, WANG M S, et al. Dual functions of zirconium modification on improving the electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2[J]. Sustainable Energy & Fuels, 2018, 2(2): 413-421. |
| 24 | LIU W, OH P, LIU X E, et al. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries[J]. Angewandte Chemie, 2015, 54(15): 4440-4457. |
| 25 | KONG D F, HU J T, CHEN Z F, et al. Ti-gradient doping to stabilize layered surface structure for high performance high-Ni oxide cathode of Li-ion battery[J]. Advanced Energy Materials, 2019, 9(41): 1901756. |
| 26 | LIU H S, ZHANG Z R, GONG Z L, et al. Origin of deterioration for LiNiO2 cathode material during storage in air[J]. Electrochemical and Solid-State Letters, 2004, 7(7): A190. |
| 27 | CHEN Z, NGUYEN H D, ZARRABEITIA M, et al. Lithium phosphonate functionalized polymer coating for high-energy Li[Ni0.8Co0.1Mn0.1]O2 with superior performance at ambient and elevated temperatures[J]. Advanced Functional Materials, 2021, 31(41): 2105343. |
| 28 | MARTINEZ A C, GRUGEON S, CAILLEU D, et al. High reactivity of the nickel-rich LiNi1- x- yMnxCoyO2 layered materials surface towards H2O/CO2 atmosphere and LiPF6-based electrolyte[J]. Journal of Power Sources, 2020, 468: 228204. |
| 29 | RENFREW S E, MCCLOSKEY B D. Residual lithium carbonate predominantly accounts for first cycle CO2 and CO outgassing of Li-stoichiometric and Li-rich layered transition-metal oxides[J]. Journal of the American Chemical Society, 2017, 139(49): 17853-17860. |
| 30 | ZHAO B, SI J, CAO C H, et al. Enhanced electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode by reducing lithium residue with low-temperature fluorination treatment[J]. Solid State Ionics, 2019, 339: 114998. |
| 31 | DOO S W, KIM K, KIM H, et al. Residual Li compounds-selective washing process for Ni-rich layered oxide cathode materials for Li-ion batteries[J]. Journal of the Electrochemical Society, 2021, 168(10): 100529. |
| 32 | YOU L Z, CHU B B, LI G X, et al. H3BO3 washed LiNi0.8Co0.1Mn0.1O2 with enhanced electrochemical performance and storage characteristics[J]. Journal of Power Sources, 2021, 482: 228940. |
| 33 | XU S, DU C Y, XU X, et al. A mild surface washing method using protonated polyaniline for Ni-rich LiNi0.8Co0.1Mn0.1O2 material of lithium ion batteries[J]. Electrochimica Acta, 2017, 248: 534-540. |
| 34 | XIONG X H, DING D, BU Y F, et al. Enhanced electrochemical properties of a LiNiO2-based cathode material by removing lithium residues with (NH4)2HPO4[J]. Journal of Materials Chemistry A, 2014, 2(30): 11691-11696. |
| 35 | LEE W, LEE S, LEE E, et al. Destabilization of the surface structure of Ni-rich layered materials by water-washing process[J]. Energy Storage Materials, 2022, 44: 441-451. |
| 36 | GU W, DONG Q Y, ZHENG L, et al. Ambient air stable Ni-rich layered oxides enabled by hydrophobic self-assembled monolayer[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 1937-1943. |
| 37 | DOO S W, LEE S, KIM H, et al. Hydrophobic Ni-rich layered oxides as cathode materials for lithium-ion batteries[J]. ACS Applied Energy Materials, 2019, 2(9): 6246-6253. |
| 38 | MU Y, MING H, CHEN X F, et al. Molecular-scale hydrophobic modification of Ni-rich cathode materials toward superior critical endurability and environmental stability[J]. Advanced Sustainable Systems, 2022, 6(6): 2200002. |
| 39 | WU L J, NAM K W, WANG X J, et al. Structural origin of overcharge-induced thermal instability of Ni-containing layered-cathodes for high-energy-density lithium batteries[J]. Chemistry of Materials, 2011, 23(17): 3953-3960. |
| 40 | ZHANG S S. Problems and their origins of Ni-rich layered oxide cathode materials[J]. Energy Storage Materials, 2020, 24: 247-254. |
| 41 | XU C, MÄRKER K, LEE J H, et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries[J]. Nature Materials, 2021, 20(1): 84-92. |
| 42 | LI S, YAO Z P, ZHENG J M, et al. Direct observation of defect-aided structural evolution in a nickel-rich layered cathode[J]. Angewandte Chemie International Edition, 2020, 59(49): 22092-22099. |
| 43 | SUN Y K, MYUNG S T, KIM M H, et al. Synthesis and characterization of Li [(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the microscale core-shell structure as the positive electrode material for lithium batteries[J]. Journal of the American Chemical Society, 2005, 127(38): 13411-13418. |
| 44 | YOON S J, PARK K J, LIM B B, et al. Improved performances of Li[Ni0.65Co0.08Mn0.27]O2 cathode material with full concentration gradient for Li-ion batteries[J]. Journal of the Electrochemical Society, 2014, 162(2): A3059-A3063. |
| 45 | MU L Q, LIN R Q, XU R, et al. Oxygen release induced chemomechanical breakdown of layered cathode materials[J]. Nano Letters, 2018, 18(5): 3241-3249. |
| 46 | YAN P F, ZHENG J M, GU M, et al. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries[J]. Nature Communications, 2017, 8(1): 1-9. |
| 47 | RYU H H, PARK K J, YOON C S, et al. Capacity fading of Ni-rich Li[NixCoyMn1- x- y]O2 (0.6≤x≤0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation?[J]. Chemistry of Materials, 2018, 30(3): 1155-1163. |
| 48 | PARK K Y, ZHU Y Z, TORRES-CASTANEDO C G, et al. Elucidating and mitigating high-voltage degradation cascades in cobalt-free LiNiO2 lithium-ion battery cathodes[J]. Advanced Materials, 2022, 34(3): 2270026. |
| 49 | XIN H L, WANG C Y. In-situ imaging of electro-chemo-mechanical degradation of high-Ni content cathode materials[J]. Microscopy and Microanalysis, 2021, 27(S1): 2180. |
| 50 | XIANG J W, WEI Y, ZHONG Y, et al. Building practical high-voltage cathode materials for lithium-ion batteries[J]. Advanced Materials, 2022, 34(52): e2200912. |
| 51 | BROUSSELY M, BIENSAN P, SIMON B. Lithium insertion into host materials: The key to success for Li ion batteries[J]. Electrochimica Acta, 1999, 45(1/2): 3-22. |
| 52 | NOH H J, YOUN S, YOON C S, et al. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x=1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries[J]. Journal of Power Sources, 2013, 233: 121-130. |
| 53 | LEE E, MUHAMMAD S, KIM T, et al. Tracking the influence of thermal expansion and oxygen vacancies on the thermal stability of Ni-rich layered cathode materials[J]. Advanced Science, 2020, 7(12): 1902413. |
| 54 | BAK S M, NAM K W, CHANG W, et al. Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode materials[J]. Chemistry of Materials, 2013, 25(3): 337-351. |
| 55 | ZHENG J M, GU M, GENC A, et al. Mitigating voltage fade in cathode materials by improving the atomic level uniformity of elemental distribution[J]. Nano Letters, 2014, 14(5): 2628-2635. |
| 56 | LEE W, MUHAMMAD S, KIM T, et al. Rechargeable batteries: New insight into Ni-rich layered structure for next-generation Li rechargeable batteries[J]. Advanced Energy Materials, 2018, 8(4): 1870015. |
| 57 | WU F, TIAN J, SU Y, et al. Effect of Ni2+ content on lithium/nickel disorder for Ni-rich cathode materials[J]. ACS Applied Materials & Interfaces, 2015, 7 (14): 7702-8. |
| 58 | AISHOVA A, PARK G T, YOON C S, et al. Cobalt-free high-capacity Ni-rich layered Li[Ni0.9Mn0.1]O2 cathode[J]. Advanced Energy Materials, 2020, 10(4): 1903179. |
| 59 | YOON C S, RYU H H, PARK G T, et al. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2 cathodes for high-energy-density lithium-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(9): 4126-4132. |
| 60 | NAKAYAMA M, KANAMORI K, NAKANO K, et al. Data-driven materials exploration for Li-ion conductive ceramics by exhaustive and informatics-aided computations[J]. The Chemical Record, 2019, 19(4): 771-778. |
| 61 | KIM M, KANG S, PARK H G, et al. Maximizing the energy density and stability of Ni-rich layered cathode materials with multivalent dopants via machine learning[J]. Chemical Engineering Journal, 2023, 452: 139254. |
| 62 | HAUTIER G, JAIN A, CHEN H L, et al. Novel mixed polyanions lithium-ion battery cathode materials predicted by high-throughput ab initio computations[J]. Journal of Materials Chemistry, 2011, 21(43): 17147-17153. |
| 63 | MIN K, CHOI B, PARK K, et al. Machine learning assisted optimization of electrochemical properties for Ni-rich cathode materials[J]. Scientific Reports, 2018, 8(1): 1-7. |
| 64 | HUA W B, WU Z G, CHEN M Z, et al. Shape-controlled synthesis of hierarchically layered lithium transition-metal oxide cathode materials by shear exfoliation in continuous stirred-tank reactors[J]. Journal of Materials Chemistry A, 2017, 5(48): 25391-25400. |
| 65 | PARK G T, SUN H H, NOH T C, et al. Nanostructured Co-free layered oxide cathode that affords fast-charging lithium-ion batteries for electric vehicles[J]. Advanced Energy Materials, 2022, 12(48): 2202719. |
| 66 | ZHANG H, HE X Z, CHEN Z H, et al. Single-crystalline Ni-rich LiNixMnyCo1- x- yO2 cathode materials: A perspective[J]. Advanced Energy Materials, 2022, 12(45): 2270192. |
| 67 | XU X, ZHU H, TANG Y, et al. Spreading monoclinic boundary network between hexagonal primary grains for high performance Ni-rich cathode materials[J]. Nano Energy, 2022, 100: 107502. |
| 68 | SALEEM A, HUSSAIN A, ASHFAQ M Z, et al. A well-controlled cracks and gliding-free single-crystal Ni-rich cathode for long-cycle-life lithium-ion batteries[J]. Journal of Alloys and Compounds, 2022, 924: 166375. |
| 69 | LIU Y, WANG Q, CHEN L, et al. Diffusion-induced stress optimization by boosted surface Li-concentration for single-crystal Ni-rich layered cathodes[J]. Materials Today, 2022, 61: 40-53. |
| 70 | CAO B K, FANG H T, LI D, et al. Controlled synthesis of single-crystalline Ni-rich cathodes for high-performance lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(48): 53667-53676. |
| 71 | XU X, HUO H, JIAN J Y, et al. Lithium-ion batteries: Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries[J]. Advanced Energy Materials, 2019, 9(15): 1970051. |
| 72 | WANG L G, LIU T C, WU T P, et al. Strain-retardant coherent perovskite phase stabilized Ni-rich cathode[J]. Nature, 2022, 611(7934): 61-67. |
| 73 | SUN Y K, MYUNG S T, PARK B C, et al. High-energy cathode material for long-life and safe lithium batteries[J]. Nature Materials, 2009, 8(4): 320-324. |
| 74 | SUN Y K, NOH H J, YOON C S. Effect of Mn content in surface on the electrochemical properties of core-shell structured cathode materials[J]. Journal of the Electrochemical Society, 2011, 159(1): A1-A5. |
| 75 | SUN Y K, KIM D H, JUNG H G, et al. High-voltage performance of concentration-gradient Li[Ni0.67Co0.15Mn0.18]O2 cathode material for lithium-ion batteries[J]. Electrochimica Acta, 2010, 55(28): 8621-8627. |
| 76 | MAENG S J, CHUNG Y, MIN S, et al. Enhanced mechanical strength and electrochemical performance of core-shell structured high-nickel cathode material[J]. Journal of Power Sources, 2020, 448: 227395. |
| 77 | KIM U H, RYU H H, KIM J H, et al. Concentration gradient cathodes: Microstructure-controlled Ni-rich cathode material by microscale compositional partition for next-generation electric vehicles[J]. Advanced Energy Materials, 2019, 9(15): 1970046. |
| 78 | PARK K J, CHOI M J, MAGLIA F, et al. High-capacity concentration gradient Li[Ni0.865Co0.120Al0.015]O2 cathode for lithium-ion batteries[J]. Advanced Energy Materials, 2018, 8(19): 1703612. |
| 79 | SUN Y K, CHEN Z H, NOH H J, et al. Nanostructured high-energy cathode materials for advanced lithium batteries[J]. Nature Materials, 2012, 11(11): 942-947. |
| 80 | SUN Y K, LEE B R, NOH H J, et al. A novel concentration-gradient Li[Ni0.83Co0.07Mn0.10]O2 cathode material for high-energy lithium-ion batteries[J]. Journal of Materials Chemistry, 2011, 21(27): 10108-10112. |
| 81 | LEE E J, CHEN Z H, NOH H J, et al. Development of microstrain in aged lithium transition metal oxides[J]. Nano Letters, 2014, 14(8): 4873-4880. |
| 82 | FENG Z J, LIU Y L, QIAN R C, et al. Fabricating a thin gradient surface layer to enhance the cycle stability of Ni-rich cathode materials[J]. Journal of Alloys and Compounds, 2022, 893: 162162. |
| 83 | JIANG Y P, LIU Z H, ZHANG Y Z, et al. Full-gradient structured LiNi0.8Co0.1Mn0.1O2 cathode material with improved rate and cycle performance for lithium ion batteries[J]. Electrochimica Acta, 2019, 309: 74-85. |
| 84 | Luo D, Ding X, Hao X, et al. Ni/Mn and Al dual concentration-gradients to mitigate voltage decay and capacity fading of Li-rich layered cathodes[J]. ACS Energy Letters, 2021, 6 (8): 2755-2764. |
| 85 | SHANG M W, CHEN X, NIU J J. Nickel-rich layered LiNi0.8Mn0.1Co0.1O2 with dual gradients on both primary and secondary particles in lithium-ion batteries[J]. Cell Reports Physical Science, 2022, 3(2): 100767. |
| 86 | SHEN Y B, YAO X J, ZHANG J H, et al. Sodium doping derived electromagnetic center of lithium layered oxide cathode materials with enhanced lithium storage[J]. Nano Energy, 2022, 94: 106900. |
| 87 | KIM S B, KIM H, PARK D H, et al. Li-ion diffusivity and electrochemical performance of Ni-rich cathode material doped with fluoride ions[J]. Journal of Power Sources, 2021, 506: 230219. |
| 88 | LIU X L, WANG S, WANG L, et al. Stabilizing the high-voltage cycle performance of LiNi0.8Co0.1Mn0.1O2 cathode material by Mg doping[J]. Journal of Power Sources, 2019, 438: 227017. |
| 89 | ZHANG D K, LIU Y, WU L, et al. Effect of Ti ion doping on electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material[J]. Electrochimica Acta, 2019, 328: 135086. |
| 90 | SRIVASTAVA N, SINGH S K, MEGHNANI D, et al. Molybdenum-doped Li/Mn-rich layered transition metal oxide cathode material Li1.2Mn0.6Ni0.1Co0.1O2 with high specific capacity and improved cyclic stability for rechargeable Li-batteries[J]. ACS Applied Energy Materials, 2022, 5(10): 12183-12195. |
| 91 | YAN J Q, HUANG H, TONG J F, et al. Recent progress on the modification of high nickel content NCM: Coating, doping, and single crystallization[J]. Interdisciplinary Materials, 2022, 1(3): 330-353. |
| 92 | LI L N, HAN E S, ZHU L Z, et al. Effect of Zr doping and Al-Zr co-doping on LiNi0.5Co0.25Mn0.25O2 for lithium-ion batteries[J]. Solid State Ionics, 2020, 346: 115220. |
| 93 | HE T, LU Y, SU Y F, et al. Sufficient utilization of zirconium ions to improve the structure and surface properties of nickel-rich cathode materials for lithium-ion batteries[J]. ChemSusChem, 2018, 11(10): 1639-1648. |
| 94 | LI Q, LI Z, WU S J, et al. Utilizing diverse functions of zirconium to enhance the electrochemical performance of Ni-rich layered cathode materials[J]. ACS Applied Energy Materials, 2020, 3(12): 11741-11751. |
| 95 | LI J W, LI Y, GUO Y N, et al. A facile method to enhance electrochemical performance of high-nickel cathode material Li(Ni0.8Co0.1Mn0.1)O2 via Ti doping[J]. Journal of Materials Science: Materials in Electronics, 2018, 29(13): 10702-10708. |
| 96 | LI G Y, HUANG Z L, ZUO Z C, et al. Understanding the trace Ti surface doping on promoting the low temperature performance of LiNi1/3Co1/3Mn1/3O2 cathode[J]. Journal of Power Sources, 2015, 281: 69-76. |
| 97 | PARK G T, RYU H H, PARK N Y, et al. Tungsten doping for stabilization of Li [Ni0.90Co0.05Mn0.05]O2 cathode for Li-ion battery at high voltage[J]. J Power Sources, 2019, 442. |
| 98 | RYU H H, PARK G T, YOON C S, et al. Suppressing detrimental phase transitions via tungsten doping of LiNiO2 cathode for next-generation lithium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(31): 18580-18588. |
| 99 | XIE Q, LI W D, MANTHIRAM A. A Mg-doped high-nickel layered oxide cathode enabling safer, high-energy-density Li-ion batteries[J]. Chemistry of Materials, 2019, 31(3): 938-946. |
| 100 | LV Y T, CHENG X, QIANG W J, et al. Improved electrochemical performances of Ni-rich LiNi0.83Co0.12Mn0.05O2 by Mg-doping[J]. Journal of Power Sources, 2020, 450: 227718. |
| 101 | FENG Z, RAJAGOPALAN R, ZHANG S, et al. A three in one strategy to achieve zirconium doping, boron doping, and interfacial coating for stable LiNi0.8Co0.1Mn0.1O2 cathode[J]. Advanced Science, 2021, 8(2): 2001809. |
| 102 | DU R, BI Y J, YANG W C, et al. Improved cyclic stability of LiNi0.8Co0.1Mn0.1O2 via Ti substitution with a cut-off potential of 4.5 V[J]. Ceramics International, 2015, 41(5): 7133-7139. |
| 103 | ZHOU Q H, LIU W H, LV L, et al. Study on d0 transition metals doped Ni-rich cathode materials for Li-ion batteries: Insights from first-principles calculations[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 656: 130421. |
| 104 | KIM U H, PARK G T, SON B K, et al. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge[J]. Nature Energy, 2020, 5(11): 860-869. |
| 105 | QIU L, ZHANG M K, SONG Y, et al. Recent advance in structure regulation of high-capacity Ni-rich layered oxide cathodes[J]. EcoMat, 2021, 3(5): doi: 10.1002/eom2.12141. |
| 106 | WOO S U, PARK B C, YOON C S, et al. Improvement of electrochemical performances of Li[Ni0.8Co0.1Mn0.1]O2 cathode materials by fluorine substitution[J]. Journal of the Electrochemical Society, 2007, 154(7): A649. |
| 107 | ZHANG B, LI L J, ZHENG J C. Characterization of multiple metals (Cr, Mg) substituted LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium ion battery[J]. Journal of Alloys and Compounds, 2012, 520: 190-194. |
| 108 | ZHANG Z, HONG B, YI M Y, et al. In situ co-doping strategy for achieving long-term cycle stability of single-crystal Ni-rich cathodes at high voltage[J]. Chemical Engineering Journal, 2022, 445: 136825. |
| 109 | PENG F, CHU Y Q, LI Y, et al. Mg, Ti-base surface integrated layer and bulk doping to suppress lattice oxygen evolution of Ni-rich cathode material at a high cut-off voltage[J]. Journal of Energy Chemistry, 2022, 71: 434-444. |
| 110 | TANG H, FU W W, XIE T, et al. High performance of phosphorus and fluorine co-doped nickel-rich cathode material for lithium ion batteries[J]. Solid State Ionics, 2021, 361: 115550. |
| 111 | KO G, PARK S, KIM W, et al. Synergistic effect of Na and Al co-doping on the electrochemical properties of Li[Ni0.8Mn0.1Co0.1]O2 cathode materials for Li-ion batteries[J]. Journal of Alloys and Compounds, 2022, 925: 166678. |
| 112 | XU C L, XIANG W, WU Z G, et al. Constructing a protective pillaring layer by incorporating gradient Mn4+ to stabilize the surface/interfacial structure of LiNi0.815Co0.15Al0.035O2 cathode[J]. ACS Applied Materials & Interfaces, 2018, 10(33): 27821-27830. |
| 113 | QIU L, XIANG W, TIAN W, et al. Polyanion and cation co-doping stabilized Ni-rich Ni-Co-Al material as cathode with enhanced electrochemical performance for Li-ion battery[J]. Nano Energy, 2019, 63: 103818. |
| 114 | ZHAO Y, LIU J T, WANG S B, et al. Surface structural transition induced by gradient polyanion-doping in Li-rich layered oxides: Implications for enhanced electrochemical performance[J]. Advanced Functional Materials, 2016, 26(26): 4760-4767. |
| 115 | ZHANG R, WANG C Y, ZOU P C, et al. Compositionally complex doping for zero-strain zero-cobalt layered cathodes[J]. Nature, 2022, 610(7930): 67-73. |
| 116 | JAMIL S, YUE L, LI C M, et al. Significance of gallium doping for high Ni, low Co/Mn layered oxide cathode material[J]. Chemical Engineering Journal, 2022, 441: 135821. |
| 117 | JUNG C H, LI Q T, KIM D H, et al. Revisiting the role of Zr doping in Ni-rich layered cathodes for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2021, 9(32): 17415-17424. |
| 118 | SUN Z H, XU L Q, DONG C Q, et al. A facile gaseous sulfur treatment strategy for Li-rich and Ni-rich cathode materials with high cycling and rate performance[J]. Nano Energy, 2019, 63: 103887. |
| 119 | LIU W, LI X F, XIONG D B, et al. Significantly improving cycling performance of cathodes in lithium ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2[J]. Nano Energy, 2018, 44: 111-120. |
| 120 | WANG W Z, WU L Y, LI Z W, et al. In situ tuning residual lithium compounds and constructing TiO2 coating for surface modification of a nickel-rich cathode toward high-energy lithium-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(12): 12423-12432. |
| 121 | MO Y, GUO L J, JIN H F, et al. Improved cycling stability of LiNi0.6Co0.2Mn0.2O2 through microstructure consolidation by TiO2 coating for Li-ion batteries[J]. Journal of Power Sources, 2020, 448: 227439. |
| 122 | MAO G H, XIAO F M, ZENG L M, et al. Improvement of cycle performance of the high nickel cathode material LiNi0.88Co0.07Al0.05O2 for lithium-ion batteries by the spray drying of V2O5[J]. Journal of Alloys and Compounds, 2022, 892: 162161. |
| 123 | LEE K S, MYUNG S T, KIM D W, et al. AlF3-coated LiCoO2 and Li[Ni1/3Co1/3Mn1/3]O2 blend composite cathode for lithium ion batteries[J]. Journal of Power Sources, 2011, 196(16): 6974-6977. |
| 124 | SONG H G, KIM S B, PARK Y J. Enhanced electrochemical properties of Li[Ni0.5Co0.2Mn0.3]O2 cathode by surface coating using LaF3 and MgF2[J]. Journal of Electroceramics, 2012, 29(2): 163-169. |
| 125 | FENG Z, RAJAGOPALAN R, SUN D, et al. In-situ formation of hybrid Li3PO4-AlPO4-Al(PO3)3 coating layer on LiNi0.8Co0.1Mn0.1O2 cathode with enhanced electrochemical properties for lithium-ion battery[J]. Chemical Engineering Journal, 2020, 382: 122959. |
| 126 | LI W, YANG L S, LI Y J, et al. Ultra-thin AlPO4 layer coated LiNi0.7Co0.15Mn0.15O2 cathodes with enhanced high-voltage and high-temperature performance for lithium-ion half/full batteries[J]. Frontiers in Chemistry, 2020, 8: 597. |
| 127 | HU G R, ZHANG Z Y, LI T F, et al. In situ surface modification for improving the electrochemical performance of Ni-rich cathode materials by using ZrP2O7[J]. ChemSusChem, 2020, 13(6): 1603-1612. |
| 128 | MA Y, XU M, ZHANG J B, et al. Improving electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode for Li-ion batteries by dual-conductive coating layer of PPy and LiAlO2[J]. Journal of Alloys and Compounds, 2020, 848: 156387. |
| 129 | GAN Q M, QIN N, ZHU Y H, et al. Polyvinylpyrrolidone-induced uniform surface-conductive polymer coating endows Ni-rich LiNi0.8Co0.1Mn0.1O2 with enhanced cyclability for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(13): 12594-12604. |
| 130 | SUN P P, DU F H, ZHOU Q, et al. Efficient preservation of surface state of LiNi0.82Co0.15Al0.03O2 through assembly of hydride terminated polydimethylsiloxane[J]. Journal of Power Sources, 2021, 495: 229761. |
| 131 | YANG S Y, SHADIKE Z, WANG W W, et al. An ultrathin solid-state electrolyte film coated on LiNi0.8Co0.1Mn0.1O2 electrode surface for enhanced performance of lithium-ion batteries[J]. Energy Storage Materials, 2022, 45: 1165-1174. |
| 132 | LEE H B, DINH HOANG T, BYEON Y S, et al. Surface stabilization of Ni-rich layered cathode materials via surface engineering with LiTaO3 for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(2): 2731-2741. |
| 133 | ZHANG Y X, KIM C S, SONG H W, et al. Ultrahigh active material content and highly stable Ni-rich cathode leveraged by oxidative chemical vapor deposition[J]. Energy Storage Materials, 2022, 48: 1-11. |
| 134 | DIAO H H, JIA M Y, ZHAO N, et al. LiNi0.6Co0.2Mn0.2O2 cathodes coated with dual-conductive polymers for high-rate and long-life solid-state lithium batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(21): 24929-24937. |
| 135 | LI Y, LIU X, REN D S, et al. Toward a high-voltage fast-charging pouch cell with TiO2 cathode coating and enhanced battery safety[J]. Nano Energy, 2020, 71: 104643. |
| 136 | CHO W, KIM S M, SONG J H, et al. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating[J]. Journal of Power Sources, 2015, 282: 45-50. |
| 137 | QIAN D N, XU B, CHO H M, et al. Lithium lanthanum titanium oxides: A fast ionic conductive coating for lithium-ion battery cathodes[J]. Chemistry of Materials, 2012, 24(14): 2744-2751. |
| 138 | CHEN H L, HE P, LI M, et al. Bifunctional sulfonated graphene-modified LiNi0.5Mn1.5O4 for long-life and high-energy-density lithium-ion batteries[J]. ACS Applied Energy Materials, 2021, 4(6): 5963-5972. |
| 139 | LEE S H, YOON C S, AMINE K, et al. Improvement of long-term cycling performance of Li[Ni0.8Co0.15Al0.05]O2 by AlF3 coating[J]. Journal of Power Sources, 2013, 234: 201-207. |
| 140 | SATTAR T, SIM S J, JIN B S, et al. Dual function Li-reactive coating from residual lithium on Ni-rich NCM cathode material for Lithium-ion batteries[J]. Scientific Reports, 2021, 11(1): 18590. |
| 141 | WU Y Q, MING H, LI M L, et al. New organic complex for lithium layered oxide modification: Ultrathin coating, high-voltage, and safety performances[J]. ACS Energy Letters, 2019, 4(3): 656-665. |
| 142 | CHEN S, HE T, SU Y F, et al. Ni-rich LiNi0.8Co0.1Mn0.1O2 oxide coated by dual-conductive layers as high performance cathode material for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(35): 29732-29743. |
| 143 | BAN H J, KIM M Y, PARK S J, et al. Electrochemical behavior of rutile phase TiO2-coated NCM materials for ASLBs operated at a high temperature[J]. Surface and Coatings Technology, 2022, 430: 127984. |
| 144 | MA B, HUANG X, LIU Z F, et al. Al2O3 coated single-crystalline hexagonal nanosheets of LiNi0.6Co0.2Mn0.2O2 cathode materials for the high-performance lithium-ion batteries[J]. Journal of Materials Science, 2022, 57(4): 2857-2869. |
| 145 | LUO Z Y, HU G R, WANG W G, et al. Enhancing the electrochemical performance of Co-less Ni-rich LiNi0.925Co0.03Mn0.045 O2 cathode material via Co-modification with Li2B4O7 coating and B3+ doping[J]. Journal of Power Sources, 2022, 548: 232092. |
| 146 | ZHANG C, LI T, XUE B, et al. Synergistic modification of Ni-rich full concentration gradient materials with enhanced thermal stability[J]. Chemical Engineering Journal, 2023, 451: 138518. |
| 147 | LV Y, HUANG S F, ZHAO Y F, et al. A review of nickel-rich layered oxide cathodes: Synthetic strategies, structural characteristics, failure mechanism, improvement approaches and prospects[J]. Applied Energy, 2022, 305: 117849. |
| 148 | JAMIL S, FASEHULLAH M, JABAR B, et al. Significantly fastened redox kinetics in single crystal layered oxide cathode by gradient doping[J]. Nano Energy, 2022, 94: 106961. |
| 149 | MO Y, GUO L J, JIN H F, et al. Building nickel-rich cathodes with large concentration gradient for high performance lithium-ion batteries[J]. Journal of Power Sources, 2020, 468: 228405. |
| 150 | SATTAR T, SIM S J, JIN B S, et al. Improving the cycle stability and rate performance of LiNi0.91Co0.06Mn0.03O2 Ni-rich cathode material by La2O3 coating for lithium-ion batteries[J]. Current Applied Physics, 2022, 36: 176-182. |
| 151 | TENG T, XIAO L, SHEN L, et al. Simultaneous Li2MoO4 coating and Mo6+ doping improves the structural stability and electrochemical properties of nickel-rich LiNi0.83Co0.11Mn0.06O2[J]. Applied Surface Science, 2022, 601: 154101. |
| [1] | Luhao HAN, Ziyang WANG, Xiaolong HE, Chunshan HE, Xiaolong SHI, Bin YAO. The effect of water mist strategies on thermal runaway fire suppression of large-capacity NCM lithium-ion battery [J]. Energy Storage Science and Technology, 2023, 12(5): 1664-1674. |
| [2] | Kangkang QU, Yahua LIU, Die HONG, Zhaoxi SHEN, Xiaozhao HAN, Xu ZHANG. Research progress on positive electrolytes for neutral aqueous organic redox flow battery [J]. Energy Storage Science and Technology, 2023, 12(5): 1570-1588. |
| [3] | Yuwen ZHAO, Huan YANG, Junpeng GUO, Yi ZHANG, Qi SUN, Zhijia ZHANG. Application of magnetic metal elements in sodium ion batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1332-1347. |
| [4] | Yongli YI, Ran YU, Wu LI, Yi JIN, Zheren DAI. Preparation of Mo, Al-doped Li7La3Zr2O12-based composite solid electrolyte and performance of all-solid-state batterys [J]. Energy Storage Science and Technology, 2023, 12(5): 1490-1499. |
| [5] | Shangzhuo LI, Yutong LONG, Zhaomeng LIU, Xuanwen GAO, Wenbin LUO. Advances toward polyanionic cathode materials for potassium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(5): 1348-1363. |
| [6] | Linze LI, Xiangwen ZHANG. SOH estimation for lithium-ion batteries based on combination of frequency impedance characteristics [J]. Energy Storage Science and Technology, 2023, 12(5): 1705-1712. |
| [7] | Ni YANG, Yuefeng SU, Lian WANG, Ning LI, Liang MA, Chen ZHU. Research progress of focused ion beam microscopy in lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(4): 1283-1294. |
| [8] | Xueli CHENG, Weifu ZHANG, Chengcheng LUO, Xiaoya YUAN. Preparation of three-dimensional graphene/Fe3O4 composites by one-step hydrothermal method and their lithium storage performance [J]. Energy Storage Science and Technology, 2023, 12(4): 1066-1074. |
| [9] | Jinhua SONG, Xinghao ZHANG, Zhenhe FENG, Guangyu CHENG, Honghui GU, Haitao GU, Ke WANG. Degradation mechanisms of SiO x -C composite anode based on in situ reference electrode [J]. Energy Storage Science and Technology, 2023, 12(4): 1059-1065. |
| [10] | Liyue HU, Xingyan YAO. Thermal runaway of lithium-ion batteries based on orthogonal test [J]. Energy Storage Science and Technology, 2023, 12(4): 1268-1277. |
| [11] | Pengkai WANG, Xinyan ZHANG, Guanghao ZHANG. Remaining useful life prediction of lithium-ion batteries based on ResNet-Bi-LSTM-Attention model [J]. Energy Storage Science and Technology, 2023, 12(4): 1215-1222. |
| [12] | Zhi ZHAI, Fujin WANG, Yi DI, Peiyu MA, Zhibin ZHAO, Xuefeng CHEN. Hierarchical alignment transfer learning for lithium-ion battery capacity estimation [J]. Energy Storage Science and Technology, 2023, 12(4): 1223-1233. |
| [13] | Kaiyuan XUE, Yan WANG, Junwei LANG, Tian HE, Zuoqiang DAI, Zongmin ZHENG. The progress in applications of dicationic ionic liquids in the energy storage and conversion system [J]. Energy Storage Science and Technology, 2023, 12(3): 808-821. |
| [14] | Kuijie LI, Ping LOU, Minyuan GUAN, Jinlong MO, Weixin ZHANG, Yuancheng CAO, Shijie CHENG. A review of multi-dimensional signal evolution and coupling mechanism of lithium-ion battery thermal runaway [J]. Energy Storage Science and Technology, 2023, 12(3): 899-912. |
| [15] | Yiming YAO, Weiling LUAN, Ying CHEN, Min SUN. Recent progress in aging degradation of lithium-ion battery materials via in-situ optical microscopy [J]. Energy Storage Science and Technology, 2023, 12(3): 777-791. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
