Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (9): 3150-3160.doi: 10.19799/j.cnki.2095-4239.2024.0586
Previous Articles Next Articles
Guobing ZHOU1,2( ), Shenzhen XU1(
), Shenzhen XU1( )
)
Received:2024-06-28
Revised:2024-07-15
Online:2024-09-28
Published:2024-09-20
Contact:
Shenzhen XU
E-mail:gbzhou@jxnu.edu.cn;xushenzhen@pku.edu.cn
CLC Number:
Guobing ZHOU, Shenzhen XU. Progress of theoretical studies on the formation and growth mechanisms of solid electrolyte interphase at lithium metal anodes[J]. Energy Storage Science and Technology, 2024, 13(9): 3150-3160.
Table 1
Comparisons of different theoretical simulation methods for SEI research"
| Simulation methods | Time/Spatial scale | Features |
|---|---|---|
| CMD | ns/nm | Advantages: high computational efficiency Disadvantages: accuracy limit, not suitable for chemical reactions |
| RxMD | ns/nm | Advantages: capable of modeling chemical reactions Disadvantages: accuracy limit, complex potential development |
| AIMD | ps/nm | Advantages: high accuracy, capable of modeling chemical reactions Disadvantages: high computational cost, limited system size |
| MLMD | ns/nm | Advantages: high computational efficiency, high accuracy, capable of modeling chemical reactions Disadvantages: transferability limitations |
| KMC | s/nm~μm | Advantages: long timescales, efficient sampling Disadvantages: event list requirement |
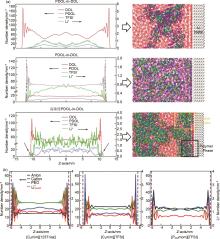
Fig. 1
(a) Number density profiles of electrolyte compositions and simulation snapshots in three different systems of PDOL-in-DOL, DOL-in-PDOL, and high-concentration PDOL-in-DOL on the Li metal surface[22]; (b) Number density profiles of electrolyte compositions in three different systems of [C2mim][123Triaz], [C2mim][TFSI] and [P222mom][TFSI] [23]"
| 1 | MASIAS A, MARCICKI J, PAXTON W A. Opportunities and challenges of lithium ion batteries in automotive applications[J]. ACS Energy Letters, 2021, 6(2): 621-630. DOI: 10.1021/acsenergylett.0c02584. |
| 2 | KIM S, PARK G, LEE S J, et al. Lithium-metal batteries: From fundamental research to industrialization[J]. Advanced Materials, 2023, 35(43): e2206625. DOI: 10.1002/adma.202206625. |
| 3 | KWAK W J, ROSY, SHARON D, et al. Lithium-oxygen batteries and related systems: Potential, status, and future[J]. Chemical Reviews, 2020, 120(14): 6626-6683. DOI: 10.1021/acs.chemrev.9b00609. |
| 4 | ZHANG X, YANG Y A, ZHOU Z. Towards practical lithium-metal anodes[J]. Chemical Society Reviews, 2020, 49(10): 3040-3071. DOI: 10.1039/c9cs00838a. |
| 5 | LIU D H, BAI Z Y, LI M, et al. Developing high safety Li-metal anodes for future high-energy Li-metal batteries: Strategies and perspectives[J]. Chemical Society Reviews, 2020, 49(15): 5407-5445. DOI: 10.1039/c9cs00636b. |
| 6 | MENG X Q, XU Y L, CAO H B, et al. Internal failure of anode materials for lithium batteries—A critical review[J]. Green Energy & Environment, 2020, 5(1): 22-36. DOI: 10.1016/j.gee. 2019.10.003. |
| 7 | YU X W, MANTHIRAM A. Electrode-electrolyte interfaces in lithium-based batteries[J]. Energy & Environmental Science, 2018, 11(3): 527-543. DOI: 10.1039/C7EE02555F. |
| 8 | ADENUSI H, CHASS G A, PASSERINI S, et al. Lithium batteries and the solid electrolyte interphase (SEI)—Progress and outlook[J]. Advanced Energy Materials, 2023, 13(10): 2203307. DOI: 10.1002/aenm.202203307. |
| 9 | GAUTHIER M, CARNEY T J, GRIMAUD A, et al. Electrode-electrolyte interface in Li-ion batteries: Current understanding and new insights[J]. The Journal of Physical Chemistry Letters, 2015, 6(22): 4653-4672. DOI: 10.1021/acs.jpclett.5b01727. |
| 10 | MENKIN S, GOLODNITSKY D, PELED E. Artificial solid-electrolyte interphase (SEI) for improved cycleability and safety of lithium-ion cells for EV applications[J]. Electrochemistry Communications, 2009, 11(9): 1789-1791. DOI: 10.1016/j.elecom.2009.07.019. |
| 11 | SHIM J, KOSTECKI R, RICHARDSON T, et al. Electrochemical analysis for cycle performance and capacity fading of a lithium-ion battery cycled at elevated temperature[J]. Journal of Power Sources, 2002, 112(1): 222-230. DOI: 10.1016/S0378-7753(02)00363-4. |
| 12 | SMITH A J, BURNS J C, TRUSSLER S, et al. Precision measurements of the coulombic efficiency of lithium-ion batteries and of electrode materials for lithium-ion batteries[J]. Journal of the Electrochemical Society, 2010, 157(2): A196. DOI: 10.1149/1.3268129. |
| 13 | SHAN X Y, ZHONG Y, ZHANG L J, et al. A brief review on solid electrolyte interphase composition characterization technology for lithium metal batteries: Challenges and perspectives[J]. The Journal of Physical Chemistry C, 2021, 125(35): 19060-19080. DOI: 10.1021/acs.jpcc.1c06277. |
| 14 | WU J X, IHSAN-UL-HAQ M, CHEN Y M, et al. Understanding solid electrolyte interphases: Advanced characterization techniques and theoretical simulations[J]. Nano Energy, 2021, 89: 106489. DOI: 10.1016/j.nanoen.2021.106489. |
| 15 | VERMA P, MAIRE P, NOVÁK P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries[J]. Electrochimica Acta, 2010, 55(22): 6332-6341. DOI: 10.1016/j.electacta.2010.05.072. |
| 16 | WU H P, JIA H, WANG C M, et al. Recent progress in understanding solid electrolyte interphase on lithium metal anodes[J]. Advanced Energy Materials, 2021, 11(5): 2003092. DOI: 10.1002/aenm.202003092. |
| 17 | 张慧敏, 王京, 王一博, 等. 锂离子电池SEI多尺度建模研究展望[J]. 储能科学与技术, 2023, 12(2): 366-382. DOI: 10.19799/j.cnki.2095-4239.2022.0504. |
| ZHANG H M, WANG J, WANG Y B, et al. Multiscale modeling of the SEI of lithium-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(2): 366-382. DOI: 10.19799/j.cnki.2095-4239.2022.0504. | |
| 18 | 于沛平, 许亮, 麻冰云, 等. 多尺度模拟研究固体电解质界面[J]. 储能科学与技术, 2022, 11(3): 921-928. DOI: 10.19799/j.cnki.2095-4239.2022.0046. |
| YU P P, XU L, MA B Y, et al. Multiscale simulation of a solid electrolyte interphase[J]. Energy Storage Science and Technology, 2022, 11(3): 921-928. DOI: 10.19799/j.cnki.2095-4239.2022.0046. | |
| 19 | TAKENAKA N, BOUIBES A, YAMADA Y, et al. Frontiers in theoretical analysis of solid electrolyte interphase formation mechanism[J]. Advanced Materials, 2021, 33(37): e2100574. DOI: 10.1002/adma.202100574. |
| 20 | WU Q S, MCDOWELL M T, QI Y. Effect of the electric double layer (EDL) in multicomponent electrolyte reduction and solid electrolyte interphase (SEI) formation in lithium batteries[J]. Journal of the American Chemical Society, 2023, 145(4): 2473-2484. DOI: 10.1021/jacs.2c11807. |
| 21 | JORN R, KUMAR R, ABRAHAM D P, et al. Atomistic modeling of the electrode-electrolyte interface in Li-ion energy storage systems: Electrolyte structuring[J]. The Journal of Physical Chemistry C, 2013, 117(8): 3747-3761. DOI: 10.1021/jp3102282. |
| 22 | KANG P B, CHEN D L, WU L Y, et al. Insight into poly(1,3-dioxolane)-based polymer electrolytes and their interfaces with lithium metal: Effect of electrolyte compositions[J]. Chemical Engineering Journal, 2023, 455: 140931. DOI: 10.1016/j.cej.2022.140931. |
| 23 | LOURENÇO T C, EBADI M, BRANDELL D, et al. Interfacial structures in ionic liquid-based ternary electrolytes for lithium-metal batteries: A molecular dynamics study[J]. The Journal of Physical Chemistry B, 2020, 124(43): 9648-9657. DOI: 10.1021/acs.jpcb.0c06500. |
| 24 | VAN DUIN A C T, DASGUPTA S, LORANT F, et al. ReaxFF: A reactive force field for hydrocarbons[J]. The Journal of Physical Chemistry A, 2001, 105(41): 9396-9409. DOI: 10.1021/jp004368u. |
| 25 | LEE H G, KIM S Y, LEE J S. Dynamic observation of dendrite growth on lithium metal anode during battery charging/discharging cycles[J]. NPJ Computational Materials, 2022, 8: 103. DOI: 10.1038/s41524-022-00788-6. |
| 26 | YANG P Y, PAO C W. Molecular simulations of the microstructure evolution of solid electrolyte interphase during cyclic charging/discharging[J]. ACS Applied Materials & Interfaces, 2021, 13(4): 5017-5027. DOI: 10.1021/acsami.0c18783. |
| 27 | XIE M, WU Y, LIU Y, et al. Pathway of in situ polymerization of 1,3-dioxolane in LiPF6 electrolyte on Li metal anode[J]. Materials Today Energy, 2021, 21: 100730. DOI: 10.1016/j.mtener. 2021.100730. |
| 28 | LIU Y, SUN Q T, YU P P, et al. Effects of high and low salt concentrations in electrolytes at lithium-metal anode surfaces using DFT-ReaxFF hybrid molecular dynamics method[J]. The Journal of Physical Chemistry Letters, 2021, 12(11): 2922-2929. DOI: 10.1021/acs.jpclett.1c00279. |
| 29 | YU P P, SUN Q T, LIU Y, et al. Multiscale simulation of solid electrolyte interface formation in fluorinated diluted electrolytes with lithium anodes[J]. ACS Applied Materials & Interfaces, 2022, 14(6): 7972-7979. DOI: 10.1021/acsami.1c22610. |
| 30 | YANG M Y, ZYBIN S V, DAS T, et al. Characterization of the solid electrolyte interphase at the Li metal-ionic liquid interface[J]. Advanced Energy Materials, 2023, 13(3): 2202949. DOI: 10.1002/aenm.202202949. |
| 31 | MERINOV B V, ZYBIN S V, NASERIFAR S, et al. Interface structure in Li-metal/[Pyr14][TFSI]-ionic liquid system from ab initio molecular dynamics simulations[J]. The Journal of Physical Chemistry Letters, 2019, 10(16): 4577-4586. DOI: 10.1021/acs.jpclett.9b01515. |
| 32 | DHATTARWAL H S, CHEN Y W, KUO J L, et al. Mechanistic insight on the formation of a solid electrolyte interphase (SEI) by an acetonitrile-based superconcentrated [Li][TFSI] electrolyte near lithium metal[J]. The Journal of Physical Chemistry C, 2020, 124(50): 27495-27502. DOI: 10.1021/acs.jpcc.0c08009. |
| 33 | GALVEZ-ARANDA D E, SEMINARIO J M. Li-metal anode in dilute electrolyte LiFSI/TMP: Electrochemical stability using ab initio molecular dynamics[J]. The Journal of Physical Chemistry C, 2020, 124(40): 21919-21934. DOI: 10.1021/acs.jpcc.0c04240. |
| 34 | ZHENG Y, SOTO F A, PONCE V, et al. Localized high concentration electrolyte behavior near a lithium-metal anode surface[J]. Journal of Materials Chemistry A, 2019, 7(43): 25047-25055. DOI: 10.1039/C9TA08935G. |
| 35 | HU T P, TIAN J X, DAI F Z, et al. Impact of the local environment on Li ion transport in inorganic components of solid electrolyte interphases[J]. Journal of the American Chemical Society, 2023, 145(2): 1327-1333. DOI: 10.1021/jacs.2c11521. |
| 36 | FU F J, WANG X X, ZHANG L F, et al. Unraveling the atomic-scale mechanism of phase transformations and structural evolutions during (de)lithiation in Si anodes[J]. Advanced Functional Materials, 2023, 33(37): 2303936. DOI: 10.1002/adfm.202303936. |
| 37 | REN F C, WU Y Q, ZUO W H, et al. Visualizing the SEI formation between lithium metal and solid-state electrolyte[J]. Energy & Environmental Science, 2024, 17(8): 2743-2752. DOI: 10.1039/D3EE03536K. |
| 38 | FANG F, LIN J, LI J J, et al. Molecular dynamics simulations of liquid gallium alloy Ga-X (X = Pt, Pd, Rh) via machine learning potentials[J]. Inorganic Chemistry Frontiers, 2024, 11(5): 1573-1582. DOI: 10.1039/D3QI02410E. |
| 39 | XU L K, SHAO W, JIN H S, et al. Data efficient and stability indicated sampling for developing reactive machine learning potential to achieve ultralong simulation in lithium-metal batteries[J]. The Journal of Physical Chemistry C, 2023, 127(50): 24106-24117. DOI: 10.1021/acs.jpcc.3c05522. |
| 40 | ESMAEILPOUR M, JANA S, LI H J, et al. A solution-mediated pathway for the growth of the solid electrolyte interphase in lithium-ion batteries[J]. Advanced Energy Materials, 2023, 13(14): 2203966. DOI: 10.1002/aenm.202203966. |
| 41 | SITAPURE N, LEE H, OSPINA-ACEVEDO F, et al. A computational approach to characterize formation of a passivation layer in lithium metal anodes[J]. AIChE Journal, 2021, 67(1): e17073. DOI: 10.1002/aic.17073. |
| 42 | GERASIMOV M, SOTO F A, WAGNER J, et al. Species distribution during solid electrolyte interphase formation on lithium using MD/DFT-parameterized kinetic Monte Carlo simulations[J]. The Journal of Physical Chemistry C, 2023, 127(10): 4872-4886. DOI: 10.1021/acs.jpcc.2c05898. |
| 43 | WAGNER-HENKE J, KUAI D C, GERASIMOV M, et al. Knowledge-driven design of solid-electrolyte interphases on lithium metal via multiscale modelling[J]. Nature Communications, 2023, 14(1): 6823. DOI: 10.1038/s41467-023-42212-7. |
| [1] | Jinbao FAN, Na LI, Yikun WU, Chunwang HE, Le YANG, Weili SONG, Haosen CHEN. Digital twin technology for energy batteries at the cell level [J]. Energy Storage Science and Technology, 2024, 13(9): 3112-3133. |
| [2] | Yajie LI, Yiping WANG, Bin CHEN, Hailong LIN, Geng ZHANG, Siqi SHI. Machine learning-assisted phase-field simulation for predicting the impact of lithium-ion transport parameters on maximum battery dendrite height and space utilization rate [J]. Energy Storage Science and Technology, 2024, 13(9): 2864-2870. |
| [3] | Ruihe XING, Suting WENG, Yejing LI, Jiayi ZHANG, Hao ZHANG, Xuefeng WANG. AI-assisted battery material characterization and data analysis [J]. Energy Storage Science and Technology, 2024, 13(9): 2839-2863. |
| [4] | Dinghong LIU, Wenkai DONG, Zhaoyang LI, Hongzhu ZHANG, Xin QI. Estimation of real-vehicle battery state of health using the RUN-GRU-attention model [J]. Energy Storage Science and Technology, 2024, 13(9): 3042-3058. |
| [5] | Congxin LI, Meiling YUE, Xintong LI, Qinghui XIONG, Xiaoyan LIU. Proton exchange membrane fuel cell aging performance prediction based on conditional neural networks [J]. Energy Storage Science and Technology, 2024, 13(9): 3094-3102. |
| [6] | Ziyu LIU, Zekun JIANG, Wei QIU, Quan XU, Yingchun NIU, Chunming XU, Tianhang ZHOU. Application of artificial intelligence in long-duration redox flow batteries storage systems [J]. Energy Storage Science and Technology, 2024, 13(9): 2871-2883. |
| [7] | Yingying XIE, Bin DENG, Yuzhi ZHANG, Xiaoxu WANG, Linfeng ZHANG. Intelligent R&D of battery design automation in the era of artificial intelligence [J]. Energy Storage Science and Technology, 2024, 13(9): 3182-3197. |
| [8] | Zhenwei ZHU, Jiawei MIAO, Xiayu ZHU, Xiaoxu WANG, Jingyi QIU, Hao ZHANG. Research progress in lithium-ion battery remaining useful life prediction based on machine learning [J]. Energy Storage Science and Technology, 2024, 13(9): 3134-3149. |
| [9] | Zhifeng HE, Yuanzhe TAO, Yonggang HU, Qicong Wang, Yong YANG. Machine learning-enhanced electrochemical impedance spectroscopy for lithium-ion battery research [J]. Energy Storage Science and Technology, 2024, 13(9): 2933-2951. |
| [10] | Chenyang ZHAO, Xiaokun YU, Yubing TAO. Preparation and characterization of modified CuO nanoparticles/n-octadecane phase change material [J]. Energy Storage Science and Technology, 2024, 13(6): 1786-1793. |
| [11] | Bingjin LI, Xiaoxia HAN, Wenjie ZHANG, Weiguo ZENG, Jinde WU. Review of the remaining useful life prediction methods for lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(4): 1266-1276. |
| [12] | Meiling WU, Lei NIU, Shiyou LI, Dongni ZHAO. Research progress on cathode prelithium additives used in lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(3): 759-769. |
| [13] | Heqing TIAN, Zhaoyang KOU, Junjie ZHOU, Yinsheng YU. Molecular dynamics simulation of structure and thermal properties of LiCl-KCl molten salt nanofluids [J]. Energy Storage Science and Technology, 2023, 12(3): 654-660. |
| [14] | Huimin ZHANG, Jing WANG, Yibo WANG, Jiaxin ZHENG, Jingyi QIU, Gaoping CAO, Hao ZHANG. Multiscale modeling of the SEI of lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(2): 366-382. |
| [15] | Dianwei FU, Cancan ZHANG, Heya NA, Guoqiang WANG, Yuting WU, Yuanwei LU. Review of the molecular dynamics of molten salt thermal physical properties [J]. Energy Storage Science and Technology, 2023, 12(12): 3873-3882. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
