Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (7): 2327-2347.doi: 10.19799/j.cnki.2095-4239.2024.0323
• Special Issue on Low Temperature Batteries • Previous Articles Next Articles
Xiang LI( ), Dezhong LIU(
), Dezhong LIU( ), Kai YUAN, Dapeng CHEN(
), Kai YUAN, Dapeng CHEN( )
)
Received:2024-04-11
Revised:2024-05-02
Online:2024-07-28
Published:2024-07-23
Contact:
Dezhong LIU, Dapeng CHEN
E-mail:742010446@qq.com;ldzone@outlook.com;dpchenhust@gmail.com
CLC Number:
Xiang LI, Dezhong LIU, Kai YUAN, Dapeng CHEN. Solid-state electrolyte for low-temperature lithium metal batteries[J]. Energy Storage Science and Technology, 2024, 13(7): 2327-2347.
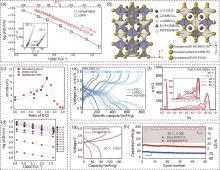
Fig. 3
(a) Arrhenius plots for the bulk conductivities of Li9.54[Si0.6Ge0.4]1.74P1.44S11.1Br0.3O0.6 and LGPS; (b) Schematic of the LSiPSBrO crystal structure determined by Rietveld analysis[34]; (c) Room-temperature ionic conductivity of chlorine-rich lithium argyrodite electrolytes prepared by the simple solid-state reaction in this work as a function of S/Cl ratios; (d) The corresponding Arrhenius plots of Li7-x PS6-x Cl x (x=1.0—2.0)[35]; (e) The charge-discharge profiles of FeS2|SE|Li-In ASSBs at second cycle and various temperatures; (f) The DRT spectra calculated from the EIS measurements of FeS2|SE | Li-In ASSBs using LASI-80Si sulfide SSEs[40]; (g) Charge/discharge curves and (h) cycling performance of pristine NCM712/Li5.5PS4.5Cl1.5/In-Li and LNO@NCM712/Li5.5PS4.5Cl1.5/In-Li SSBs between 2.4 and 3.7 V under -20 ℃ at 0.05C[44]"

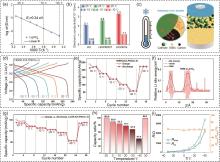
Fig. 4
(a) Arrhenius plots of LIC SSEs derived from the EIS measurements in a range of -30 ℃ to 35 ℃; (b) Electronic conductivity of LIC, LIC/PEDOT composite, and LIC/CNTs composite at 25℃ and -10 ℃; (c) Schematic illustration of Li+ and electron transfer in composite cathode at low temperatures[47]; (d) The charge-discharge curves of the second cycle and (e) the evolution of specific capacity for Ni90/LIC/LPSC/Li-In ASSBs within the temperature range of -30—30 ℃; (f) Li site energy distribution along the z-axis direction for Ni90-Li3InCl6 interfaces[49]; (g) The evolution of specific capacity for LCO/LIC/LPSC/Li-In ASSBs over the temperature range from 30 ℃ to -40 ℃; (h) The capacity ratio of LCO/LIC/LPSC/Li-In ASSBs at each temperature to that at 30 ℃; (i) R-T relationship obtained by equivalent circuit fitting of Nyquist plots for LCO/LIC/LPSC/Li-In ASSBs[50]"
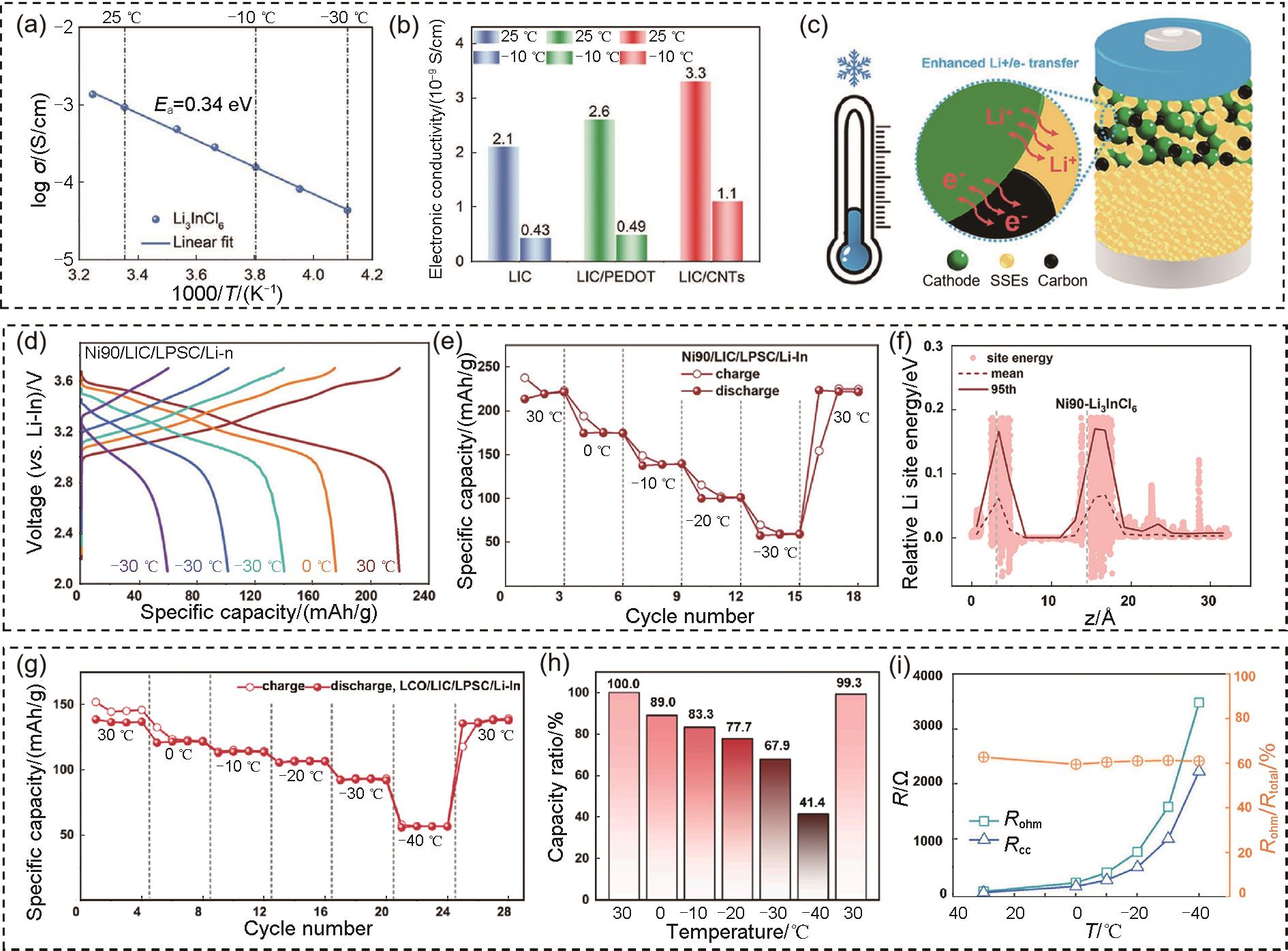

Fig. 5
(a) Variation of the ionic conductivity at room temperature of PEO-based solid-state electrolytes (Li+ ∶EO = 1∶32) with different amounts of added SN; (b) Arrhenius plots of the ionic conductivity of various solid-state electrolytes; (c) Carbon conformation transition diagram of PEO, PEO32, and Homo-SPE[55]; (d) Ionic conductivity of BStSi and PEO SPE, the inset is AC impedance spectra of BStSi; (e) Cycle performance of the LFP/BStSi/Li batteries[56]; (f) Schematic illustration of NCM particle-electrolyte solid interface in GPE and MPS-GPE at low temperatures[57]"

Fig. 6
(a) The ionic conductivity gradient of the developed electrolytes at 23 ℃ on the ternary phase diagram[59]; (b) The optimized geometric configurations and electrostatic potential of SN, ETPTA, and ETPTA-SN; (c) The binding energy of ETPTA-SN, PEO-SN, and SN-SN[67]; (d) Comparison of the melting points and viscosity (25 ℃) of various solvents; (e) HOMO and LUMO energies for commonly used Li salts, solvents, TXE monomer, PEO, and POM polymers[68]; (f) Comparison of the binding energies of pristine Li2S4-Li2S, and Li2S4-Li2S4 in PEO and IDCN electrolyte environments as calculated by DFT method; (g) Schematic showing the anode-electrolyte interface and SEI structure formed in IDCN (left) and LE (right)[69]"
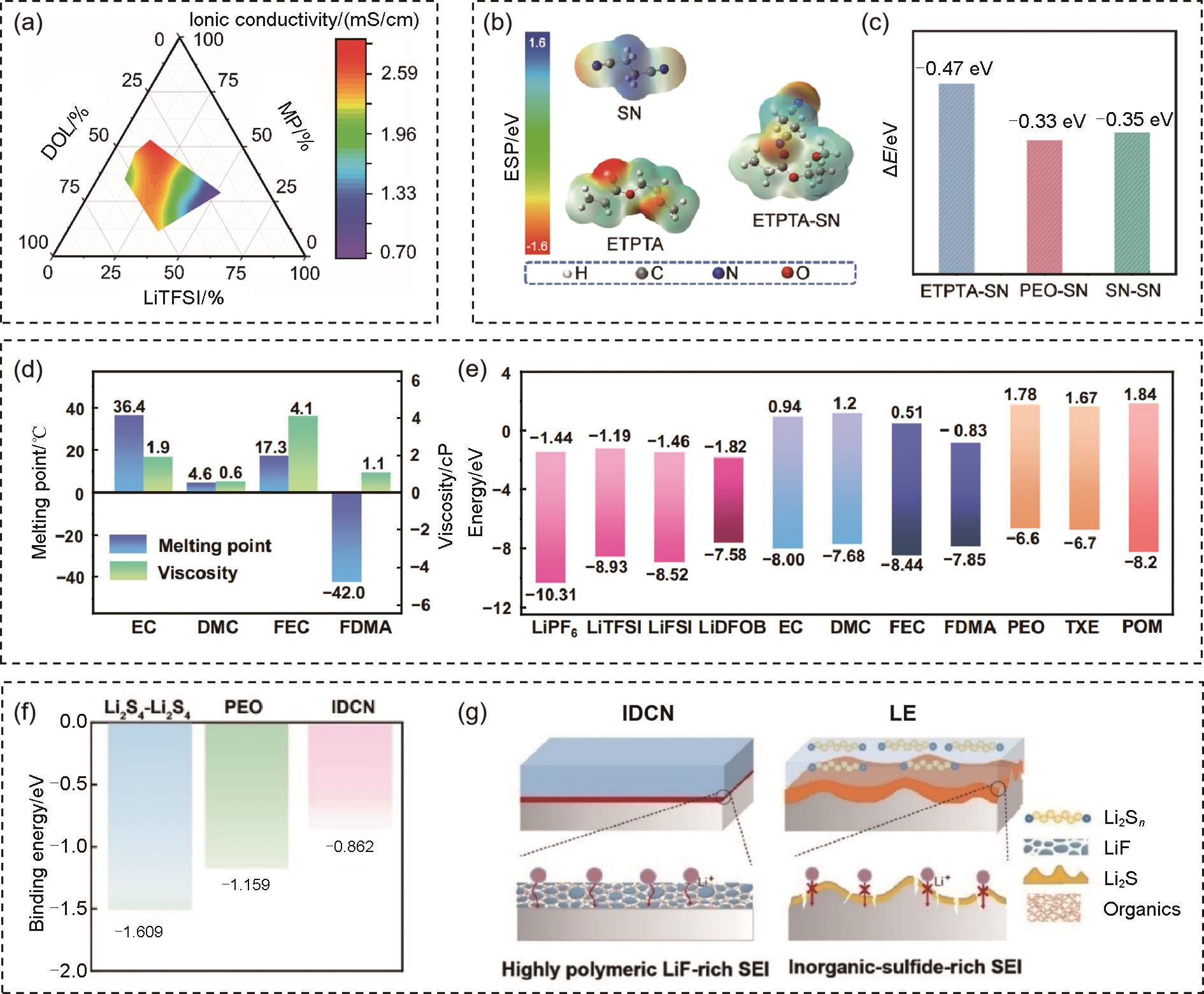

Fig. 7
(a) Proposed ion pathways in es-CGPEs[71]; (b) Cycling performance of PTNB@Li||NCM811 cell over a wide temperature range (-10—30 ℃)[73]; (c) Li-ion transport mechanism of PVLN-15 electrolyte; (d) Multiple Li-ion transport channels in PVLN-15 electrolyte[74]; (e) Temperature dependence of ionic conductivity of CSEs membranes; (f) Lithium-ion transference number of CSEs[76]; (g) The adsorption energy of SN molecule on LLTZO surfaces; (h) The adsorption energy of SN molecule on Li metal surface[77]"
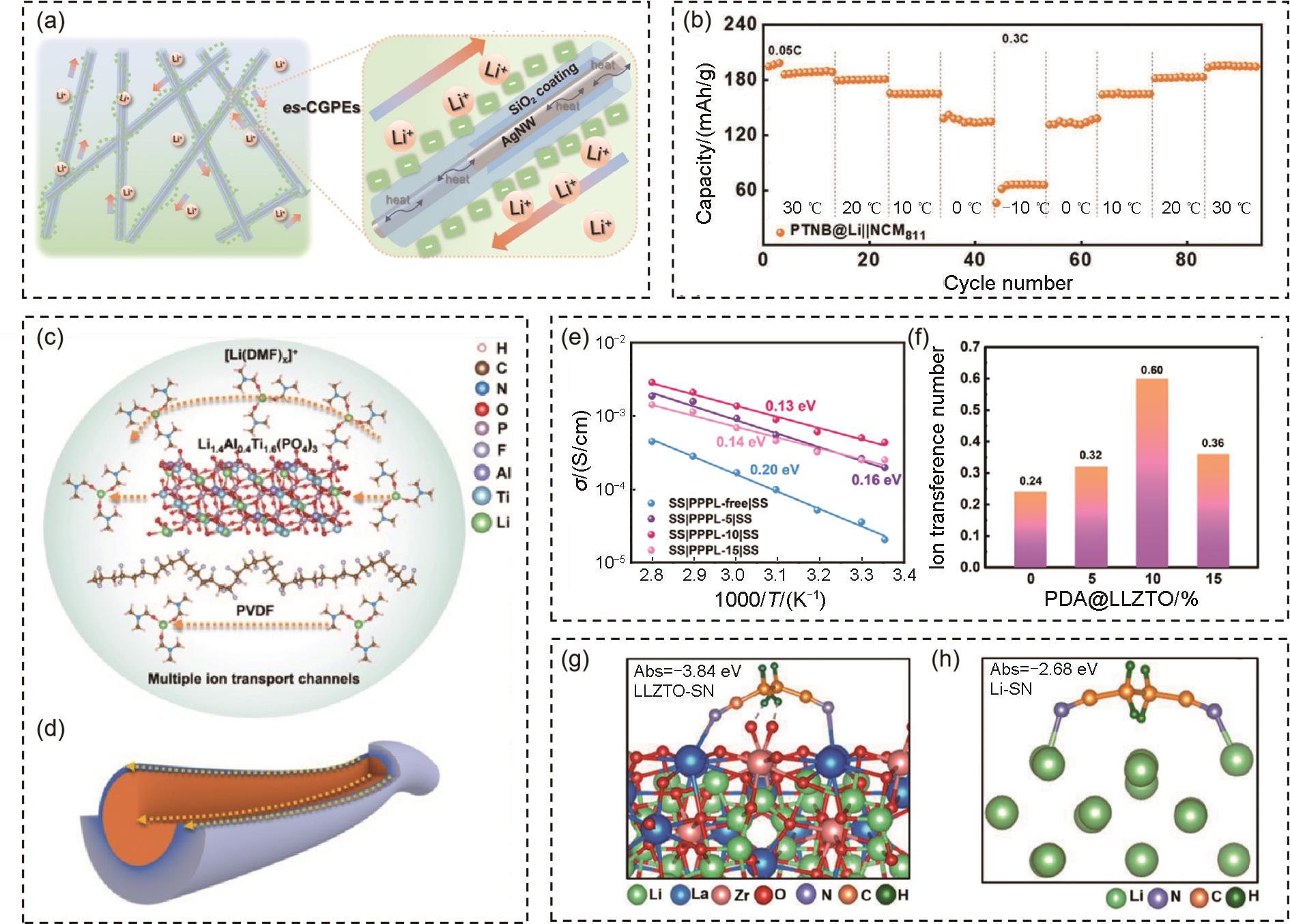

Fig. 8
(a) Schematic diagram of the SPE2[83]; (b) EIS measurements and (c) line fitting plot for σ of the PP6LS20@GF electrolyte ranging from -20 to 70 ℃; (d) ESP comparison of PEO-b-PA, SN and TFSI- anion[85]; (e) Modeling total electrolyte salt concentration for the CMP/VAMMT, CMP/MMT, pure CMP (top) and corresponding mapping of electrolyte potential distribution (bottom)[86]; (f) Galvanostatic cycle performance of the pouch cells with the Li metal anode and LiFePO4 cathode at 5 mA/cm2; (g) Illustration of pouch cells lighting light-emitting diode[87]"
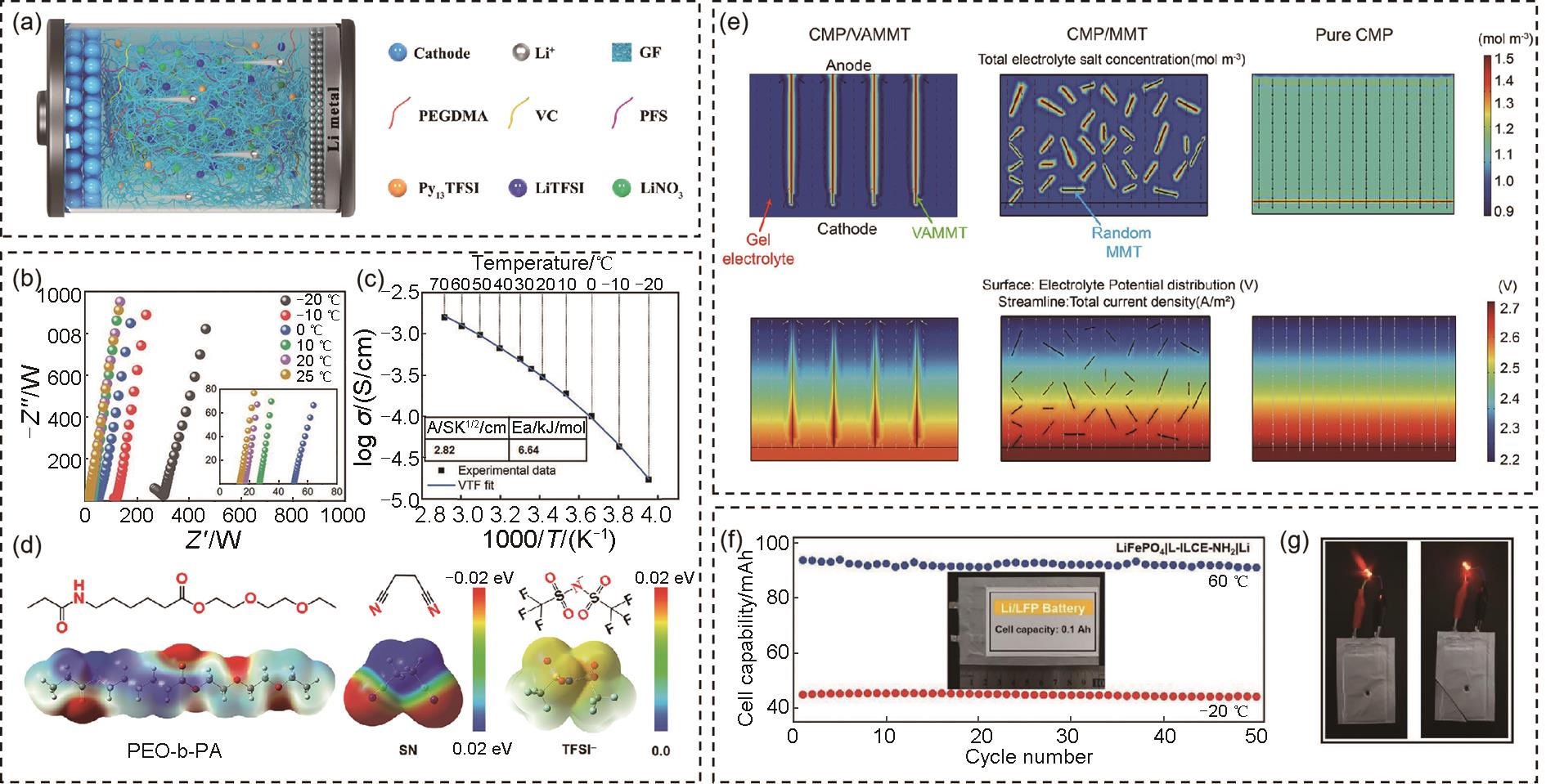
Fig. 9
(a) Illustration of UiO-Li@SPE-based LMB assembly; (b) Li deposition pathways of LMB with UiO-Li@SPE[93]; (c) Simulated structure of the LiTFSI and UiO-66 surface at different times; (d) Diagrammatic drawing of the interaction mechanism between UiO-66 and LiTFSI in the as-prepared MMSE[94]; (e) Arrhenius plots of the three as-prepared materials: LiCON-1, LiCON-2, and LiCON-3; (f) Li ion transference number of LiCONs; (g) Representative Debye plots of imaginary impedance as a function of log f[95]"

| 1 | WANG Z X, SUN Z H, LI J, et al. Insights into the deposition chemistry of Li ions in nonaqueous electrolyte for stable Li anodes[J]. Chemical Society Reviews, 2021, 50(5): 3178-3210. |
| 2 | LIU B, ZHANG J G, XU W. Advancing lithium metal batteries[J]. Joule, 2018, 2(5): 833-845. |
| 3 | XIANG J W, YANG L Y, YUAN L X, et al. Alkali-metal anodes: From lab to market[J]. Joule, 2019, 3(10): 2334-2363. |
| 4 | 许卓, 郑莉莉, 陈兵, 等. 固态电池复合电解质研究综述[J]. 储能科学与技术, 2021, 10(6): 2117-2126. |
| XU Z, ZHENG L L, CHEN B, et al. Overview of research on composite electrolytes for solid-state batteries[J]. Energy Storage Science and Technology, 2021, 10(6): 2117-2126. | |
| 5 | XIA S X, WU X S, ZHANG Z C, et al. Practical challenges and future perspectives of all-solid-state lithium-metal batteries[J]. Chem, 2019, 5 (4): 753-785. |
| 6 | SHEN Y B, ZHANG Y T, HAN S J, et al. Unlocking the energy capabilities of lithium metal electrode with solid-state electrolytes[J]. Joule, 2018, 2(9): 1674-1689. |
| 7 | CHEN S Q, WEI X Z, ZHANG G X, et al. All-temperature area battery application mechanism, performance, and strategies[J]. Innovation (Cambridge (Mass)), 2023, 4(4): 100465. |
| 8 | LI Z H, YAO Y X, SUN S, et al. 40 years of low-temperature electrolytes for rechargeable lithium batteries[J]. Angewandte Chemie (International Ed in English), 2023, 62(37): e202303888. |
| 9 | WANG C Y, ZHANG G S, GE S H, et al. Lithium-ion battery structure that self-heats at low temperatures[J]. Nature, 2016, 529: 515-518. |
| 10 | ZHANG G S, GE S H, XU T, et al. Rapid self-heating and internal temperature sensing of lithium-ion batteries at low temperatures[J]. Electrochimica Acta, 2016, 218: 149-155. |
| 11 | LI S, KIRKALDY N, ZHANG C, et al. Optimal cell tab design and cooling strategy for cylindrical lithium-ion batteries[J]. Journal of Power Sources, 2021, 492: 229594. |
| 12 | LIN J Y, LIU X H, LI S, et al. A review on recent progress, challenges and perspective of battery thermal management system[J]. International Journal of Heat and Mass Transfer, 2021, 167: 120834. |
| 13 | XIA H R, ZHANG W, CAO S K, et al. A figure of merit for fast-charging Li-ion battery materials[J]. ACS Nano, 2022, 16(6): 8525-8530. |
| 14 | ZHANG D M, MENG X L, HOU W Y, et al. Solid polymer electrolytes: Ion conduction mechanisms and enhancement strategies[J]. Nano Research Energy, 2023, 2: e9120050. |
| 15 | YANG T Q, WANG C, ZHANG W K, et al. A critical review on composite solid electrolytes for lithium batteries: Design strategies and interface engineering[J]. Journal of Energy Chemistry, 2023, 84: 189-209. |
| 16 | KOBAYASHI T, OHNISHI T, OSAWA T, et al. In-operando lithium-ion transport tracking in an all-solid-state battery[J]. Small, 2022, 18(46): 2204455. |
| 17 | LU P S, ZHOU Z M, XIAO Z X, et al. Materials and chemistry design for low-temperature all-solid-state batteries[J]. Joule, 2024, 8(3): 635-657. |
| 18 | CHAE O B, LUCHT B L. Interfacial issues and modification of solid electrolyte interphase for Li metal anode in liquid and solid electrolytes[J]. Advanced Energy Materials, 2023, 13(14): 2203791. |
| 19 | LIU J, YUAN H, LIU H, et al. Unlocking the failure mechanism of solid state lithium metal batteries[J]. Advanced Energy Materials, 2022, 12(4): 2100748. |
| 20 | FAMPRIKIS T, CANEPA P, DAWSON J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials, 2019, 18: 1278-1291. |
| 21 | CAO D X, ZHANG K N, LI W, et al. Nondestructively visualizing and understanding the mechano-electro-chemical origins of "soft short" and "creeping" in all-solid-state batteries[J]. Advanced Functional Materials, 2023, 33(52): 2307998. |
| 22 | SUN H M, LIU Q N, CHEN J Z, et al. In situ visualization of lithium penetration through solid electrolyte and dead lithium dynamics in solid-state lithium metal batteries[J]. ACS Nano, 2021, 15(12): 19070-19079. |
| 23 | 赵永智, 陈晨阳, 刘文燚, 等. 固态锂电池界面优化策略的研究进展[J]. 物理化学学报, 2023, 39(8): 45-61. |
| ZHAO Y Z, CHEN C Y, LIU W Y, et al. Research progress of interface optimization strategies for solid-state lithium batteries[J]. Acta Physico-Chimica Sinica, 2023, 39(8): 45-61. | |
| 24 | JIAO Y, WANG F, MA Y H, et al. Challenges and advances on low-temperature rechargeable lithium-sulfur batteries[J]. Nano Research, 2023, 16(6): 8082-8096. |
| 25 | WU D X, CHEN L Q, LI H, et al. Solid-state lithium batteries-from fundamental research to industrial progress[J]. Progress in Materials Science, 2023, 139: 101182. |
| 26 | KRAUSKOPF T, RICHTER F H, ZEIER W G, et al. Physicochemical concepts of the lithium metal anode in solid-state batteries[J]. Chemical Reviews, 2020, 120(15): 7745-7794. |
| 27 | YU G X, WANG Y P, LI K, et al. Plasma optimized Li7La3Zr2O12 with vertically aligned ion diffusion pathways in composite polymer electrolyte for stable solid-state lithium metal batteries[J]. Chemical Engineering Journal, 2022, 430: 132874. |
| 28 | FENG W L, ZHAO Y F, XIA Y Y. Solid interfaces for the garnet electrolytes[J]. Advanced Materials, 2024, 36(15): e2306111. |
| 29 | SONG H C, WANG S, SONG X Y, et al. Solar-driven all-solid-state lithium-air batteries operating at extreme low temperatures[J]. Energy & Environmental Science, 2020, 13(4): 1205-1211. |
| 30 | WANG S, SONG H C, SONG X Y, et al. An extra-wide temperature all-solid-state lithium-metal battery operating from -73 ℃ to 120 ℃[J]. Energy Storage Materials, 2021, 39: 139-145. |
| 31 | SONG X Y, WANG M, WANG S, et al. A wide temperature solid-state Li-S battery enabled by a plasmon-enhanced copper–silicon nanowire photothermal current collector[J]. Journal of Materials Chemistry A, 2022, 10(42): 22584-22591. |
| 32 | ZHANG Q, CAO D X, MA Y, et al. Sulfide-based solid-state electrolytes: Synthesis, stability, and potential for all-solid-state batteries[J]. Advanced Materials, 2019, 31(44): 1901131. |
| 33 | KAMAYA N, HOMMA K, YAMAKAWA Y, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10: 682-686. |
| 34 | LI Y X, SONG S B, KIM H, et al. A lithium superionic conductor for millimeter-thick battery electrode[J]. Science, 2023, 381(6653): 50-53. |
| 35 | PENG L F, YU C, ZHANG Z Q, et al. Chlorine-rich lithium argyrodite enabling solid-state batteries with capabilities of high voltage, high rate, low-temperature and ultralong cyclability[J]. Chemical Engineering Journal, 2022, 430: 132896. |
| 36 | PENG L F, YU C, ZHANG Z Q, et al. Tuning solid interfaces via varying electrolyte distributions enables high‐performance solid‐state batteries[J]. Energy & Environmental Materials, 2023, 6 (2): e12308. |
| 37 | LU P S, XIA Y, SUN G C, et al. Realizing long-cycling all-solid-state Li-In||TiS2 batteries using Li6+ xMxAs1- xS5I (M=Si, Sn) sulfide solid electrolytes[J]. Nature Communications, 2023, 14: 4077. |
| 38 | WANG R, WU Z K, YU C, et al. Low temperature ensures FeS2 cathode a superior cycling stability in Li7P3S11-based all-solid-state lithium batteries[J]. Frontiers in Energy Research, 2023, 10: 1108789. |
| 39 | LU P S, GONG S, GUO F L, et al. Amorphous bimetallic polysulfide for all-solid-state batteries with superior capacity and low-temperature tolerance[J]. Nano Energy, 2023, 118: 109029. |
| 40 | LU P S, XIA Y, HUANG Y L, et al. Wide-temperature, long-cycling, and high-loading pyrite all-solid-state batteries enabled by argyrodite thioarsenate superionic conductor[J]. Advanced Functional Materials, 2023, 33(8): 2211211. |
| 41 | WANG Y P, YUAN P C, XU Z Y, et al. Ti3C2T MXene in situ transformed Li2TiO3 interface layer enabling 4.5 V-LiCoO2/sulfide all-solid-state lithium batteries with superior rate capability and cyclability[J]. Chinese Chemical Letters, 2024, 35(6): 108776. |
| 42 | KATO Y, HORI S, SAITO T, et al. High-power all-solid-state batteries using sulfide superionic conductors[J]. Nature Energy, 2016, 1(4): 16030. |
| 43 | MORINO Y. Impact of surface coating on the low temperature performance of a sulfide-based all-solid-state battery cathode[J]. Electrochemistry, 2022, 90(2): 027001. |
| 44 | PENG L F, REN H T, ZHANG J Z, et al. LiNbO3-coated LiNi0.7Co0.1Mn0.2O2 and chlorine-rich argyrodite enabling high-performance solid-state batteries under different temperatures[J]. Energy Storage Materials, 2021, 43: 53-61. |
| 45 | ZHU Y Z, HE X F, MO Y F. Origin of outstanding stability in the lithium solid electrolyte materials: Insights from thermodynamic analyses based on first-principles calculations[J]. ACS Applied Materials & Interfaces, 2015, 7(42): 23685-23693. |
| 46 | 李枫, 程晓斌, 罗锦达, 等. 金属氯化物固态电解质及其全固态电池研究现状与展望[J]. 储能科学与技术, 2024, 13(1): 193-211. |
| LI F, CHENG X B, LUO J D, et al. Metal chloride solid-state electrolytes and all-solid-state batteries: State-of-the-art developments and perspectives[J]. Energy Storage Science and Technology, 2024, 13(1): 193-211. | |
| 47 | DENG S X, JIANG M, CHEN N, et al. Regulating electronic conductivity at cathode interface for low-temperature halide-based all-solid-state batteries[J]. Advanced Functional Materials, 2022, 32(45): 2205594. |
| 48 | ZHANG Z C, JIA W Q, FENG Y, et al. An ultraconformal chemo-mechanical stable cathode interface for high-performance all-solid-state batteries at wide temperatures[J]. Energy & Environmental Science, 2023, 16(10): 4453-4463. |
| 49 | LU P S, GONG S, WANG C H, et al. Superior low-temperature all-solid-state battery enabled by high-ionic-conductivity and low-energy-barrier interface[J]. ACS Nano, 2024, 18(10): 7334-7345. |
| 50 | LU P S, WU Y J, WU D X, et al. Rate-limiting mechanism of all-solid-state battery unravelled by low-temperature test-analysis flow[J]. Energy Storage Materials, 2024, 67: 103316. |
| 51 | WANG C H, LIANG J W, KIM J T, et al. Prospects of halide-based all-solid-state batteries: From material design to practical application[J]. Science Advances, 2022, 8(36): eadc9516. |
| 52 | SHEN M N, WANG Z Y, CHENG D M, et al. Molecular regulated polymer electrolytes for solid-state lithium metal batteries: Mechanisms and future prospects[J]. eTransportation, 2023, 18: 100264. |
| 53 | PAN L, FENG S F, SUN H, et al. Ultrathin, mechanically durable, and scalable polymer-in-salt solid electrolyte for high-rate lithium metal batteries[J]. Small, 2024: e2400272. |
| 54 | DING P, LIN Z, GUO X, et al. Polymer electrolytes and interfaces in solid-state lithium metal batteries[J]. Materials Today, 2021, 51: 449-474. |
| 55 | XU S J, SUN Z H, SUN C G, et al. Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature[J]. Advanced Functional Materials, 2020, 30(51): 2007172. |
| 56 | LIN Z H, LIU J. Low-temperature all-solid-state lithium-ion batteries based on a di-cross-linked starch solid electrolyte[J]. RSC Advances, 2019, 9(59): 34601-34606. |
| 57 | MO S K, AN H W, LIU Q S, et al. Multistage bridge engineering for electrolyte and interface enables quasi-solid batteries to operate at -40℃[J]. Energy Storage Materials, 2024, 65: 103179. |
| 58 | LI Z, FU J L, ZHOU X Y, et al. Ionic conduction in polymer-based solid electrolytes[J]. Advanced Science, 2023, 10(10): e2201718. |
| 59 | YU J, LIN X D, LIU J P, et al. In situ fabricated quasi-solid polymer electrolyte for high-energy-density lithium metal battery capable of subzero operation[J]. Advanced Energy Materials, 2021, 12 (2): 2102932. |
| 60 | XIANG J W, ZHANG Y, ZHANG B, et al. A flame-retardant polymer electrolyte for high performance lithium metal batteries with an expanded operation temperature[J]. Energy & Environmental Science, 2021, 14(6): 3510-3521. |
| 61 | HU A Y, LIAO Z, HUANG J, et al. In-situ construction of dual lithium-ion migration channels in polymer electrolytes for lithium metal batteries[J]. Chemical Engineering Journal, 2022, 448: 137661. |
| 62 | REN W, ZHANG Y, LV R, et al. In-situ formation of quasi-solid polymer electrolyte for improved lithium metal battery performances at low temperatures[J]. Journal of Power Sources, 2022, 542: 231773. |
| 63 | LI M J, YANG J X, SHI Y Q, et al. Soluble organic cathodes enable long cycle life, high rate, and wide-temperature lithium-ion batteries[J]. Advanced Materials, 2022, 34(5): e2107226. |
| 64 | LIN Z Y, GUO X W, WANG Z C, et al. A wide-temperature superior ionic conductive polymer electrolyte for lithium metal battery[J]. Nano Energy, 2020, 73: 104786. |
| 65 | WANG D Y, JIN B Y, REN Y Y, et al. Bifunctional solid-state copolymer electrolyte with stabilized interphase for high-performance lithium metal battery in a wide temperature range[J]. ChemSusChem, 2022, 15(16): e202200993. |
| 66 | YU L, YU L, LIU Q, et al. Monolithic task-specific ionogel electrolyte membrane enables high-performance solid-state lithium-metal batteries in wide temperature range[J]. Advanced Functional Materials, 2021, 32 (14): 2110653. |
| 67 | WANG A X, GENG S X, ZHAO Z, et al. In situ cross‐linked plastic crystal electrolytes for wide‐temperature and high‐energy‐density lithium metal batteries[J]. Advanced Functional Materials, 2022, 32 (28): 2201861. |
| 68 | LI Z, YU R, WENG S T, et al. Tailoring polymer electrolyte ionic conductivity for production of low-temperature operating quasi-all-solid-state lithium metal batteries[J]. Nature Communications, 2023, 14: 482. |
| 69 | ZHANG J, CHOU J, LUO X X, et al. A fully amorphous, dynamic cross-linked polymer electrolyte for lithium-sulfur batteries operating at subzero-temperatures[J]. Angewandte Chemie (International Ed in English), 2024, 63(5): e202316087. |
| 70 | LIANG H M, WANG L, WANG A P, et al. Tailoring practically accessible polymer/inorganic composite electrolytes for all-solid-state lithium metal batteries: A review[J]. Nano-Micro Letters, 2023, 15(1): 42. |
| 71 | GAN H H, YUAN J L, ZHANG Y, et al. Electrospun composite gel polymer electrolytes with high thermal conductivity toward wide temperature lithium metal batteries[J]. ACS Applied Energy Materials, 2021, 4 (8): 8130-8141. |
| 72 | LV F, LIU K X, WANG Z Y, et al. Ultraviolet-cured polyethylene oxide-based composite electrolyte enabling stable cycling of lithium battery at low temperature[J]. Journal of Colloid and Interface Science, 2021, 596: 257-266. |
| 73 | CHEN Z, KIM G T, KIM J K, et al. Highly stable quasi-solid-state lithium metal batteries: Reinforced Li1.3Al0.3Ti1.7(PO4)3/Li interface by a protection interlayer[J]. Advanced Energy Materials, 2021, 11(30): 2101339. |
| 74 | YANG K, CHEN L K, MA J B, et al. Stable interface chemistry and multiple ion transport of composite electrolyte contribute to ultra-long cycling solid-state LiNi0.8Co0.1Mn0.1O2/lithium metal batteries[J]. Angewandte Chemie (International Ed in English), 2021, 60(46): 24668-24675. |
| 75 | ZHANG Z Y, ZHANG S, GENG S X, et al. Agglomeration-free composite solid electrolyte and enhanced cathode-electrolyte interphase kinetics for all-solid-state lithium metal batteries[J]. Energy Storage Materials, 2022, 51: 19-28. |
| 76 | WANG Y, CHEN Z, WU Y X, et al. PVDF-HFP/PAN/PDA@LLZTO composite solid electrolyte enabling reinforced safety and outstanding low-temperature performance for quasi-solid-state lithium metal batteries[J]. ACS Applied Materials & Interfaces, 2023, 15(17): 21526-21536. |
| 77 | ZHANG X Y, FU C K, CHENG S C, et al. Novel PEO-based composite electrolyte for low-temperature all-solid-state lithium metal batteries enabled by interfacial cation-assistance[J]. Energy Storage Materials, 2023, 56: 121-131. |
| 78 | DE KLERK N J J, WAGEMAKER M. Space-charge layers in all-solid-state batteries; important or negligible?[J]. ACS Applied Energy Materials, 2018, 1(10): 5609-5618. |
| 79 | SHI P R, MA J B, LIU M, et al. A dielectric electrolyte composite with high lithium-ion conductivity for high-voltage solid-state lithium metal batteries[J]. Nature Nanotechnology, 2023, 18: 602-610. |
| 80 | CUAN J, ZHOU Y, ZHOU T F, et al. Borohydride-scaffolded Li/Na/Mg fast ionic conductors for promising solid-state electrolytes[J]. Advanced Materials, 2019, 31(1): e1803533. |
| 81 | HU C J, SHEN Y B, SHEN M, et al. Superionic conductors via bulk interfacial conduction[J]. Journal of the American Chemical Society, 2020, 142(42): 18035-18041. |
| 82 | WEI Y Q, LI Z L, CHEN Z C, et al. A wide temperature 10 V solid-state electrolyte with a critical current density of over 20 mA·cm-2[J]. Energy & Environmental Science, 2023, 16(10): 4679-4692. |
| 83 | LI J, ZHANG H T, CUI Y Y, et al. Constructing interfacial gradient layers and enhancing lithium salt dissolution kinetics for high-rate solid-state batteries[J]. Nano Energy, 2022, 102: 107716. |
| 84 | LI J, CAI Y J, CUI Y Y, et al. Fabrication of asymmetric bilayer solid-state electrolyte with boosted ion transport enabled by charge-rich space charge layer for -20~70 ℃ lithium metal battery[J]. Nano Energy, 2022, 95: 107027. |
| 85 | HUANG X, HUANG S, WANG T, et al. Polyether-b-amide based solid electrolytes with well-adhered interface and fast kinetics for ultralow temperature solid-state lithium metal batteries[J]. Advanced Functional Materials, 2023, 33 (27): 2300683. |
| 86 | LI X Y, WANG Y, XI K, et al. Quasi-solid-state ion-conducting arrays composite electrolytes with fast ion transport vertical-aligned interfaces for all-weather practical lithium-metal batteries[J]. Nano-Micro Letters, 2022, 14(1): 210. |
| 87 | ZHANG Y F, HUANG J J, LIU H, et al. Lamellar ionic liquid composite electrolyte for wide-temperature solid-state lithium-metal battery[J]. Advanced Energy Materials, 2023, 13 (23): 2300156. |
| 88 | WANG X, DU T, DONG X, et al. Application of advanced wide-temperature range and flame retardant "leaf-vein" structured functionality composite quasi-solid-state electrolyte[J]. Energy Storage Materials, 2024, 68: 103355 |
| 89 | ZHENG Y, YAO Y Z, OU J H, et al. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures[J]. Chemical Society Reviews, 2020, 49(23): 8790-8839. |
| 90 | ZHU F L, BAO H F, WU X S, et al. High-performance metal-organic framework-based single ion conducting solid-state electrolytes for low-temperature lithium metal batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(46): 43206-43213. |
| 91 | LI D, WANG J, GUO S, et al. Molecular-scale interface engineering of metal-organic frameworks toward ion transport enables high-performance solid lithium metal battery[J]. Advanced Functional Materials, 2020, 30 (50): 2003945. |
| 92 | ZHANG Q, LI D X, WANG J, et al. Multiscale optimization of Li-ion diffusion in solid lithium metal batteries via ion conductive metal-organic frameworks[J]. Nanoscale, 2020, 12(13): 6976-6982. |
| 93 | LIU Y, WANG S, JIANG Z, et al. Multifunctional asymmetric electrolyte membrane encouraging durable lithium-metal batteries in wide temperature variations[J]. Journal of Membrane Science, 2023, 677: 121636. |
| 94 | YAO M, YU T H, RUAN Q Q, et al. High-voltage and wide-temperature lithium metal batteries enabled by ultrathin MOF-derived solid polymer electrolytes with modulated ion transport[J]. ACS Applied Materials & Interfaces, 2021, 13(39): 47163-47173. |
| 95 | LI X, HOU Q, HUANG W, et al. Solution-processable covalent organic framework electrolytes for all-solid-state Li-organic batteries[J]. ACS Energy Letters, 2020, 5 (11): 3498-3506. |
| [1] | Changhao LI, Shuping WANG, Xiankun YANG, Ziqi ZENG, Xinyue ZHOU, Jia XIE. Nonaqueous electrolyte in low-temperature lithium-ion battery [J]. Energy Storage Science and Technology, 2024, 13(7): 2286-2299. |
| [2] | Yang LU, Shuaishuai YAN, Xiao MA, Zhi LIU, Weili ZHANG, Kai LIU. Low-temperature electrolytes and their application in lithium batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2224-2242. |
| [3] | Shijie LIAO, Ying WEI, Yunhui HUANG, Renzong HU, Henghui XU. 1,3-Difluorobenzene diluent-stabilizing electrode interface for high-performance low-temperature lithium metal batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2124-2130. |
| [4] | Jiaqi HUANG, Jieming XIONG, Enzhong TAN, Xinyu SUN, Liwei CHENG, Hua WANG. Revisiting the Na metal half-cell at low-temperature [J]. Energy Storage Science and Technology, 2024, 13(7): 2151-2160. |
| [5] | Xiongwen XU, Ying MO, Wang ZHOU, Huandong YAO, Juan HONG, Hua LEI, Jian TU, Jilei LIU. Effect of hard carbon kinetic properties on low-temperature performance of Na-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2141-2150. |
| [6] | Weiqi LIN, Qiaoyu LU, Yuhong CHEN, Linyuan QIU, Yurong JI, Lianyu GUAN, Xiang DING. Advances in cathode materials for low-temperature sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2348-2360. |
| [7] | Zheng LI, Zhenzhong YANG, Qiong WANG, Renzong HU. Patent intelligence analysis of the research progress in low-temperature electrolytes for Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2317-2326. |
| [8] | Lifeng WANG, Naiqing REN, Hai YANG, Yu YAO, Yan YU. Advances in low-temperature electrolytes for sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2206-2223. |
| [9] | Yuhao WANG, Zhiyong LI, Xin GUO. Applications and challenges of polymer-based electrolytes in low-temperature solid-state lithium batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2243-2258. |
| [10] | Zeheng LI, Lei XU, Yuxing YAO, Chong YAN, Ximin ZHAI, Xuechun HAO, Aibing CHEN, Jiaqi HUANG, Xiaofei BIE, Huanli SUN, Lizhen FAN, Qiang ZHANG. A review of electrolyte reducing lithium plating in low-temperature lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2192-2205. |
| [11] | Shuping WANG, Xiankun YANG, Changhao LI, Ziqi ZENG, Yifeng CHENG, Jia XIE. Diethyl ethylphosphonate-based flame-retardant wide-temperature-range electrolyte in lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2161-2170. |
| [12] | Lulu NIE, Lige YUAN. Application and optimization of intelligent electronic control system in low temperature battery management [J]. Energy Storage Science and Technology, 2024, 13(7): 2432-2434. |
| [13] | Hui FANG. Functional safety guarantee strategy for low temperature lithium battery energy storage system under network analysis mode [J]. Energy Storage Science and Technology, 2024, 13(7): 2447-2449. |
| [14] | Junjie LU, Dan PENG, Wenjing NI, Yuan YANG, Jinglun WANG. Research progress on electrolyte for Li/CF x battery [J]. Energy Storage Science and Technology, 2024, 13(5): 1487-1495. |
| [15] | Qianqian ZHANG. Application of phase change storage technology in food cold chain logistics [J]. Energy Storage Science and Technology, 2024, 13(2): 480-482. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
