储能科学与技术 ›› 2023, Vol. 12 ›› Issue (4): 1093-1109.doi: 10.19799/j.cnki.2095-4239.2022.0720
汤才1( ), 蒋江民1(
), 蒋江民1( ), 王新峰1, 刘广发1, 崔艳华2(
), 王新峰1, 刘广发1, 崔艳华2( ), 庄全超1(
), 庄全超1( )
)
收稿日期:2022-12-02
修回日期:2023-01-14
出版日期:2023-04-05
发布日期:2023-05-08
通讯作者:
蒋江民,崔艳华,庄全超
E-mail:1585112441@qq.com;jiangmin326@163.com;cuiyanhua@netease.com;zhuangquanchao@126.com
作者简介:汤才(1997—),男,硕士研究生,研究方向为氟化碳基锂一次电池的电极/电解液界面特性,E-mail:1585112441@qq.com;
基金资助:
Cai TANG1( ), Jiangmin JIANG1(
), Jiangmin JIANG1( ), Xinfeng WANG1, Guangfa LIU1, Yanhua CUI2(
), Xinfeng WANG1, Guangfa LIU1, Yanhua CUI2( ), Quanchao ZHUANG1(
), Quanchao ZHUANG1( )
)
Received:2022-12-02
Revised:2023-01-14
Online:2023-04-05
Published:2023-05-08
Contact:
Jiangmin JIANG, Yanhua CUI, Quanchao ZHUANG
E-mail:1585112441@qq.com;jiangmin326@163.com;cuiyanhua@netease.com;zhuangquanchao@126.com
摘要:
锂/氟化碳(Li/CF x )一次电池是目前能量密度最高的化学电源,具有输出电压稳定、安全性好、使用温度范围宽和自放电率低等特点,在军事(单兵作战系统)、医疗(心脏起搏器)、太空探索(空间站)等关键领域具有无可替代的重要性。然而,氟化碳材料的电子导电性较差,很大程度地影响了电化学反应的电极过程动力学,导致Li/CF x 一次电池存在高倍率放电性能差、初始放电电压延迟严重、放电过程中发热量大等问题。本文通过对近期相关文献的探讨,首先综述了Li/CF x 一次电池在放电机理方面的研究进展,包括两相放电反应机理模型、生成石墨层间化合物中间相的放电反应机理模型、“核-壳”模型反应机理和边缘传播放电反应机理以及最近刚被提出的三步放电反应机理等。其次,重点分析了Li/CF x 一次电池面临问题的解决方法,包括氟化碳材料前驱体的选择、氟化方法的改进、复合材料的构建以及电解液的改性和优化方法。其中,氟化碳纳米管、氟化富勒烯、氟化石墨烯等新型氟化碳基材料的应用为氟化碳的发展提供了新的前景。在复合材料的构建策略上,导电聚合物、金属纳米颗粒、氧化物的加入可显著降低电压延迟时间和提升倍率性能。在电解液的调控策略上,氟离子结合剂的引入和氟化锂晶体生长动力学的计算,对于溶解氟化锂和控制氟化锂的生长具有重要作用,有望实现兼具高能量密度和高功率密度的宽温域Li/CF x 一次电池。
中图分类号:
汤才, 蒋江民, 王新峰, 刘广发, 崔艳华, 庄全超. Li/CF x 一次电池研究进展[J]. 储能科学与技术, 2023, 12(4): 1093-1109.
Cai TANG, Jiangmin JIANG, Xinfeng WANG, Guangfa LIU, Yanhua CUI, Quanchao ZHUANG. Research progress of Li/CF x primary batteries[J]. Energy Storage Science and Technology, 2023, 12(4): 1093-1109.
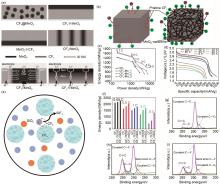
图8
(a) 四种不同构型的CF x -MnO2 复合阴极(CF x @MnO2 、CF x ⊕MnO2 、CF x ⊕MnO2 和CF x //MnO2 )的排列示意图,以及CF x //MnO2 的电荷转移机制[72];(b) CF x 和CF x @MnO2 纳米线复合材料的电子和锂离子路径示意图;(c) 原始CF x 和CF x @MnO2-纳米线复合材料的Ragone图;(d) CF x /MnO2-纳米线复合材料在0.1 C时的高低温放电曲线[74];(e) CF x 与SiO2 反应机理示意图;(f) 不同电流密度下的各种基于CF x 正极的质量能量密度;(g) CF x 、(h) CF x -TEOS和 (i) CF x -mSiO2 的高分辨率C1s光谱[75]"

| 1 | 陈淼淼, 邵钦君, 陈剑. 锂电池高比能量正极材料Cr8O21的制备及应用[J]. 储能科学与技术, 2022, 11(9): 3011-3020. |
| CHEN M M, SHAO Q J, CHEN J. Preparation and application of Cr8O21 as cathode material for high specific energy lithium batteries[J]. Energy Storage Science and Technology, 2022, 11(9): 3011-3020. | |
| 2 | XIAO Y K, JIAN J H, FU A, et al. Substantially promoted energy density of Li||CFx primary battery enabled by Li+-DMP coordinated structure[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(19): 6217-6229. |
| 3 | BAN J, JIAO X X, FENG Y Y, et al. All-temperature, high-energy-density Li/CFx batteries enabled by a fluorinated ether as a cosolvent[J]. ACS Applied Energy Materials, 2021, 4(4): 3777-3784. |
| 4 | RUFF O, BRETSCHNEIDER O. Die reaktionsprodukte der verschiedenen kohlenstoffformen mitfluor ii[J]. Zeitschrift fü r anorganische und allgemeine Chemie, 1934, 217(1):1-18. |
| 5 | ZHANG Q, TAKEUCHI K J, TAKEUCHI E S, et al. Progress towards high-power Li/CFx batteries: Electrode architectures using carbon nanotubes with CFx[J]. Physical Chemistry Chemical Physics: PCCP, 2015, 17(35): 22504-22518. |
| 6 | 夏金童,陈小华,征茂平等.新型固体润滑剂──氟化石墨的制备与性能的研究[J].无机材料学报,1998(03):435-439. |
| XIA J T, CHEN X H, ZHENG M P, et al. New Type of the solid lubricant material-Preparation and properties of graphite fluoride[J]. Journal of Inorganic Materials, 1998, (03): 435-439. | |
| 7 | GUPTA V, NAKAJIMA T, OHZAWA Y. Fluorination of graphite at high temperatures[J]. Collection of Czechoslovak Chemical Communications, 2002, 67(9): 1366-1372. |
| 8 | GIRAUDET J, CLAVES D, GUÉRIN K, et al. Magnesium batteries: Towards a first use of graphite fluorides[J]. Journal of Power Sources, 2007, 173(1): 592-598. |
| 9 | NEWMAN G H, SHROPSHIRE J A. Primary cell for electric batteries: US3990915[P]. 1976-11-09. |
| 10 | GROULT H, TRESSAUD A. Use of inorganic fluorinated materials in lithium batteries and in energy conversion systems[J]. Chemical Communications (Cambridge, England), 2018, 54(81): 11375-11382. |
| 11 | GUÉRIN K, DUBOIS M, HOUDAYER A, et al. Applicative performances of fluorinated carbons through fluorination routes: A review[J]. Journal of Fluorine Chemistry, 2012, 134: 11-17. |
| 12 | 王俊, 张学全, 刘亚飞, 等. 高容量富锂锰基正极材料的研究进展[J]. 储能科学与技术, 2022, 11(10): 3051-3061. |
| WANG J, ZHANG X Q, LIU Y F, et al. Research progress of high capacity Li-Mn-rich cathode materials[J]. Energy Storage Science and Technology, 2022, 11(10): 3051-3061. | |
| 13 | ZHANG H M, LI X F, ZHANG H Z. Li-S and Li-O2 batteries with high specific energy[M]// Li-S and Li-O2 batteries with high specific energy. Singapore: Springer, 2017: 1-48. |
| 14 | BRUCE P G, FREUNBERGER S A, HARDWICK L J, et al. Li-O2 and Li-S batteries with high energy storage[J]. Nature Materials, 2012, 11(1): 19-29. |
| 15 | YAO L, ZHOU H, CHEN G, et al. Study on the thermal effect in ultra-fast plasma-enhanced fluorination for high-quality CFx cathode materials[J]. Diamond and Related Materials, 2023, 131: doi: 10.1016/j.diamond.2022.109545. |
| 16 | GOMADAM P M, MERRITT D R, SCOTT E R, et al. Modeling lithium/hybrid-cathode batteries[J]. Journal of Power Sources, 2007, 174(2): 872-876. |
| 17 | 陈雨晴, 张洪章, 于滢, 等. 锂硫一次电池的研究现状及展望[J]. 储能科学与技术, 2017, 6(3): 529-533. |
| CHEN Y Q, ZHANG H Z, YU Y, et al. The R & D status and prospects for primary lithium sulfur batteries[J]. Energy Storage Science and Technology, 2017, 6(3): 529-533. | |
| 18 | ZHU D L, YUAN J C, DAI Y, et al. High-rate performance of fluorinated carbon material doped by phosphorus species for lithium-fluorinated carbon battery[J]. Energy Technology, 2022, 10(6): doi: 10.1002/ente.202200155. |
| 19 | GIRAUDET J, DELABARRE C, GUÉRIN K, et al. Comparative performances for primary lithium batteries of some covalent and semi-covalent graphite fluorides[J]. Journal of Power Sources, 2006, 158(2): 1365-1372. |
| 20 | CHEN K M, MERRITT D R, HOWARD W G, et al. Hybrid cathode lithium batteries for implantable medical applications[J]. Journal of Power Sources, 2006, 162(2): 837-840. |
| 21 | DREWS J, FEHRMANN G, STAUB R, et al. Primary batteries for implantable pacemakers and defibrillators[J]. Journal of Power Sources, 2001, 97: 747-749. |
| 22 | RODRIGUEZ M A, KEENAN M R, NAGASUBRAMANIAN G. in situ X-ray diffraction analysis of (CFx) nbatteries: Signal extraction by multivariate analysis[J]. Journal of Applied Crystallography, 2007, 40(6): 1097-1104. |
| 23 | WHITACRE J F, WEST W C, SMART M C, et al. Enhanced low-temperature performance of Li-CFx batteries[J]. Electrochemical and Solid-State Letters, 2007, 10(7): A166. |
| 24 | WAITTIN GHAM MS.Mechanism of reduction of the fluorographite cathode[J]. Journal of the Electrochemical Society, 1975, 122(4): 526. |
| 25 | WATANABE N, HAGIWARA R, NAKAJIMA T, et al. Solvents effects on electrochemical characteristics of graphite fluoride—Lithium batteries[J]. Electrochimica Acta, 1982, 27(11): 1615-1619. |
| 26 | WATANABE N, NAKAJIMA T, HAGIWARA R. Discharge reaction and overpotential of the graphite fluoride cathode in a nonaqueous lithium cell[J]. Journal of Power Sources, 1987, 20(1/2): 87-92. |
| 27 | ZHANG S S, FOSTER D, WOLFENSTINE J, et al. Electrochemical characteristic and discharge mechanism of a primary Li/CFx cell[J]. Journal of Power Sources, 2009, 187(1): 233-237. |
| 28 | ZHONG G M, CHEN H X, CHENG Y, et al. Insights into the lithiation mechanism of CFx by a joint high-resolution 19F NMR, in situ TEM and 7Li NMR approach[J]. Journal of Materials Chemistry A, 2019, 7(34): 19793-19799. |
| 29 | LEUNG K, SCHORR N B, MAYER M, et al. Edge-propagation discharge mechanism in CFx batteries-a first-principles and experimental study[J]. Chemistry of Materials, 2021, 33(5):1760-1770. |
| 30 | SAYAHPOUR B, HIRSH H, BAI S, et al. Revisiting discharge mechanism of CFx as a high energy density cathode material for lithium primary battery[J]. Advanced Energy Materials, 2022, 12(5): doi: 10.1002/aenm.202103196. |
| 31 | GUÉRIN K, PINHEIRO J P, DUBOIS M, et al. Synthesis and characterization of highly fluorinated graphite containing sp2 and sp3 carbon[J]. Chemistry of Materials, 2004, 16(9): 1786-1792. |
| 32 | 百华. 氟化石墨纤维[J]. 化工新型材料, 1988, 16(11): 25-28. |
| BAI H. Fluorinated graphite fiber[J]. New Chemical Materials, 1988, 16(11): 25-28. | |
| 33 | CHAMSSEDINE F, DUBOIS M, GUERIN K, et al. Reactivity of carbon nanofibers with fluorine gas[J]. Chemistry of Materials, 2007, 19(2):161-172. |
| 34 | ZHOU R X, LI Y, FENG Y Y, et al. The electrochemical performances of fluorinated hard carbon as the cathode of lithium primary batteries[J]. Composites Communications, 2020, 21: doi: 10.1016/j.coco.2020.100396. |
| 35 | AHMAD Y, DUBOIS M, GUERIN K, et al. High energy density of primary lithium batteries working with sub-fluorinated few walled carbon nanotubes cathode[J]. Journal of Alloys and Compounds, 2017, 726: 852-859. |
| 36 | JAYASINGHE R, THAPA A K, DHARMASENA R R, et al. Optimization of multi-walled carbon nanotube based CFx electrodes for improved primary and secondary battery performances[J]. Journal of Power Sources, 2014, 253: 404-411. |
| 37 | NAKAJIMA T, MATSUO Y, ŽEMVA B, et al. Synthesis of fluorine-graphite intercalation compounds by elemental fluorine and high oxidation-state transition-metal fluorides[J]. Carbon, 1996, 34(12): 1595-1598. |
| 38 | CLAVES D, GIRAUDET A J, HAMWI A, et al. Structural, bonding, and electrochemical properties of perfluorinated fullerene C70[J]. Journal of Physical Chemistry B, 2001, 105: 1739-1742. |
| 39 | HAMWI A. Fluorine reactivity with graphite and fullerenes[J]. Journal of Physics & Chemistry of Solids, 1996, 57(6-8):677-688. |
| 40 | MEDURI P, CHEN H H, XIAO J, et al. Tunable electrochemical properties of fluorinated graphene[J]. Journal of Materials Chemistry A, 2013, 1(27): 7866-7869. |
| 41 | DAMIEN D, SUDEEP P M, NARAYANAN T N, et al. Fluorinated graphene based electrodes for high performance primary lithium batteries[J]. RSC Advances, 2013, 3(48): 25702-25706. |
| 42 | BI X, LI Y Y, QIU Z P, et al. Fluorinated graphene prepared by direct fluorination of N, O-doped graphene aerogel at different temperatures for lithium primary batteries[J]. Materials (Basel, Switzerland), 2018, 11(7): 1072. |
| 43 | WATANABE N. Two types of graphite fluorides, (CF)n and (C2F)n, and discharge characteristics and mechanisms of electrodes of (CF)n and (C2F)n in lithium batteries[J]. Solid State Ionics, 1980, 1(1/2): 87-110. |
| 44 | YAZAMI R, HAMWI A. A new graphite fluoride compound as electrode material for lithium intercalation in solid state cells[J]. Solid State Ionics, 1988, 28: 1756-1761. |
| 45 | HAGIWARA R, LERNER M, BARTLETT N, et al. A lithium/C2F primary battery[J]. Journal of The Electrochemical Society, 1988, 135(9): 2393-2394. |
| 46 | NAKAJIMA T, KOH M, GUPTA V, et al. Electrochemical behavior of graphite highly fluorinated by high oxidation state complex fluorides and elemental fluorine[J]. Electrochimica Acta, 2000, 45(10): 1655-1661. |
| 47 | SATO Y, ITOH K, HAGIWARA R, et al. On the so-called "semi-ionic" C-F bond character in fluorine-GIC[J]. Carbon, 2004, 42(15): 3243-3249. |
| 48 | HANY P, YAZAMI R, HAMWI A. Low-temperature carbon fluoride for high power density lithium primary batteries[J]. Journal of Power Sources, 1997, 68(2): 708-710. |
| HANY P, YAZAMI R, HAMWI A. Low-temperature carbon fluoride for high power density lithium primary batteries[J]. Journal of Power Sources, 1997, 68(2): 708-710. | |
| 49 | NAZAROV A S, MAKOTCHENKO V G. Dicarbon monofluoride: A solid host for containment of volatiles[J]. Inorganic Materials, 2002, 38(3): 278-282. |
| 50 | GUPTA V, NAKAJIMA T, OHZAWA Y, et al. A study on the formation mechanism of graphite fluorides by Raman spectroscopy[J]. Journal of Fluorine Chemistry, 2003, 120(2): 143-150. |
| 51 | AMATUCCI G G, PEREIRA N. Fluoride based electrode materials for advanced energy storage devices[J]. Journal of Fluorine Chemistry, 2007, 128(4): 243-262. |
| 52 | READ J A, BEHL W K. Mechanochemical synthesis of carbon fluorides[J]. Electrochemical and Solid State Letters, 2008, 12(1): A16-A18. |
| 53 | NAKAJIMA T. Fluorine compounds as energy conversion materials[J]. Journal of Fluorine Chemistry, 2013, 149: 104-111. |
| 54 | DELABARRE C, DUBOIS M, GIRAUDET J, et al. Electrochemical performance of low temperature fluorinated graphites used as cathode in primary lithium batteries[J]. Carbon, 2006, 44(12): 2543-2548. |
| 55 | FULVIO P F, BROWN S S, ADCOCK J, et al. Low-temperature fluorination of soft-templated mesoporous carbons for a high-power lithium/carbon fluoride battery[J]. Chemistry of Materials, 2011, 23(20):4420-4427. |
| 56 | LEROUX F, DUBOIS M. Origin of the highly enhanced porosity of styryl LDH hybrid-type carbon replicas and study of a subsequent fluorination at low-temperature[J]. Journal of Materials Chemistry, 2006, 16(46): 4510-4520. |
| 57 | PARMENTIER J, SCHLIENGER S, DUBOIS M, et al. Structural/textural properties and water reactivity of fluorinated activated carbons[J]. Carbon, 2012, 50(14): 5135-5147. |
| 58 | MATEI GHIMBEU C, GUERIN K, DUBOIS M, et al. Insights on the reactivity of ordered porous carbons exposed to different fluorinating agents and conditions[J]. Carbon, 2015, 84: 567-583. |
| 59 | 夏金童, 征茂平, 何莉萍, 等. 氟化石墨制备新工艺的研究[J]. 湖南大学学报(自然科学版), 1999, 26(1): 29-32. |
| XIA J T, ZHENG M P, HE L P, et al. A study on the preparation of graphite fluoride by the new technology[J]. Journal of Hunan University (Natural Science), 1999, 26(1): 29-32. | |
| 60 | YAZAMI R, HAMWI A, GUÉRIN K, et al. Fluorinated carbon nanofibres for high energy and high power densities primary lithium batteries[J]. Electrochemistry Communications, 2007, 9(7): 1850-1855. |
| 61 | ADCOCK J L, FULVIO P F, DAI S. Towards the selective modification of soft-templated mesoporous carbon materials by elemental fluorine for energy storage devices[J]. Journal of Materials Chemistry A, 2013, 1(33): 9327-9331. |
| 62 | FULVIO P F, VEITH G M, ADCOCK J L, et al. Fluorination of âbrick and mortarâ soft-templated graphitic ordered mesoporous carbons for high power lithium-ion battery[J]. Journal of Materials Chemistry A, 2013, 1(33): 9414-9417. |
| 63 | CHEN G T, ZHOU H P, ZHANG S, et al. Surface de-fluorination and bond modification of CFx by high-density hydrogen plasma processing[J]. ACS Applied Energy Materials, 2021, 4(8): 8615-8620. |
| 64 | DAI Y, CAI S D, WU L J, et al. Surface modified CFx cathode material for ultrafast discharge and high energy density[J]. Journal of Materials Chemistry A, 2014, 2(48): 20896-20901. |
| 65 | GROULT H, JULIEN C M, BAHLOUL A, et al. Improvements of the electrochemical features of graphite fluorides in primary lithium battery by electrodeposition of polypyrrole[J]. Electrochemistry Communications, 2011, 13(10): 1074-1076. |
| 66 | LI L, ZHU L, PAN Y, et al. Integrated polyaniline-coated CFx cathode materials with enhanced electrochemical capabilities for Li/CFx primary battery[J]. International Journal of Electrochemical Science, 2016, 11(8):6838-6847. |
| 67 | YIN X D, LI Y, FENG Y Y, et al. Polythiophene/graphite fluoride composites cathode for high power and energy densities lithium primary batteries[J]. Synthetic Metals, 2016, 220: 560-566. |
| 68 | WANG X Z, HO J W, YANG Q Y, et al. Performance enhancement in organic photovoltaic devices using plasma-polymerized fluorocarbon-modified Ag nanoparticles[J]. Organic Electronics, 2011, 12(11): 1943-1947. |
| 69 | ZHANG L X, ZHANG L J, XILI D G, et al. CFx-Cu composites with excellent high rate performances as cathode materials for lithium primary batteries[J]. Rare Metal Materials and Engineering, 2020, 49(7):2256-2261. |
| 70 | ZHANG L X, ZHANG L J, XI L D G. Facile fabrication of CFx-Pt composites as a high-performance cathode for primary lithium batteries[J]. International Journal of Electrochemical Science, 2019, 14(6):5738-5747. |
| 71 | ZHANG L X, ZHANG L J, XILI D G, et al. CFx-Ru composite cathode for lithium primary battery with significantly improved electrochemical performance[J]. Chinese Journal of Inorganic Chemistry, 2020, 36(1):148-158. |
| 72 | LI Y, FENG W. The tunable electrochemical performances of carbon fluorides/Manganese dioxide hybrid cathodes by their arrangements[J]. Journal of Power Sources, 2015, 274: 1292-1299. |
| 73 | CHANG Y L, WANG M, WANG S P, et al. Ultralong storage life of Li/MnO2 primary batteries using MnO2-(CFx)n with C-F semi-ionic bond as cathode materials[J]. Electrochimica Acta, 2019, 320: doi: 10.1016/j.electacta.2019.134618 |
| 74 | LUO Z Y, WAN J, LEI W X, et al. A simple strategy to synthesis CFx@MnO2-nanowires composite cathode materials for high energy density and high power density primary lithium batteries[J]. Materials Technology, 2020, 35(13/14): 836-842. |
| 75 | ZHU Y L, ZHANG L J, ZHAO H H, et al. Significantly improved electrochemical performance of CFx promoted by SiO2 modification for primary lithium batteries[J]. Journal of Materials Chemistry A, 2017, 5(2): 796-803. |
| 76 | NAGASUBRAMANIAN G, SANCHEZ B. A new chemical approach to improving discharge capacity of Li/(CFx)n cells[J]. Journal of Power Sources, 2007, 165(2): 630-634. |
| 77 | LI Q, XUE W R, SUN X R, et al. Gaseous electrolyte additive BF3 for high-power Li/CFx primary batteries[J]. Energy Storage Materials, 2021, 38: 482-488. |
| 78 | IGNATOVA A A, YARMOLENKO O V, TULIBAEVA G Z, et al. Influence of 15-crown-5 additive to a liquid electrolyte on the performance of Li/CFx-Systems at temperatures up to -50 ℃[J]. Journal of Power Sources, 2016, 309: 116-121. |
| 79 | LONG S T, CHEN F, DING F, et al. Study on crystal growth kinetics and preferred orientation for LiF crystal in dimethyl sulfoxide/1, 3-dioxolane-based electrolyte[J]. The Journal of Physical Chemistry C, 2019, 123(46): 28048-28057. |
| 80 | FANG Z, YANG Y, ZHENG T L, et al. An all-climate CFx/Li battery with mechanism-guided electrolyte[J]. Energy Storage Materials, 2021, 42: 477-483. |
| [1] | 王跃迪, 仇中柱, 吴渺, 朱燕艳, 屈蒙. 多孔NiMoO4/NiCo2S4 复合材料的制备及其电化学性能[J]. 储能科学与技术, 2023, 12(4): 1034-1044. |
| [2] | 封迈, 陈楠, 陈人杰. 锂离子电池低温电解液的研究进展[J]. 储能科学与技术, 2023, 12(3): 792-807. |
| [3] | 祝玉婷, 闫共芹, 林羽芊. MoS2/RGO复合材料的电化学性能和第一性原理研究[J]. 储能科学与技术, 2023, 12(3): 698-709. |
| [4] | 薛凯元, 汪妍, 郎俊伟, 何田, 戴作强, 郑宗敏. 双阳离子型离子液体在能量存储和转化体系中的应用进展[J]. 储能科学与技术, 2023, 12(3): 808-821. |
| [5] | 王泽峥, 曲文浩, 王亚军, 秦润, 柳亦兵. 大容量复合材料飞轮转子仿真与应力分析[J]. 储能科学与技术, 2023, 12(3): 669-675. |
| [6] | 张群斌, 董陶, 李晶晶, 刘艳侠, 张海涛. 废旧电池电解液回收及高值化利用研发进展[J]. 储能科学与技术, 2022, 11(9): 2798-2810. |
| [7] | 欧宇, 侯文会, 刘凯. 锂离子电池中的智能安全电解液研究进展[J]. 储能科学与技术, 2022, 11(6): 1772-1787. |
| [8] | 房茂霖, 张英, 乔琳, 刘淑敏, 曹中琦, 张华民, 马相坤. 铁铬液流电池技术的研究进展[J]. 储能科学与技术, 2022, 11(5): 1358-1367. |
| [9] | 方亮, 张凯, 周丽敏. 铝离子电池电解液的研究进展[J]. 储能科学与技术, 2022, 11(4): 1236-1245. |
| [10] | 王心怡, 李维杰, 韩朝, 刘化鹍, 窦世学. 水系锌离子电池金属负极的挑战与优化策略[J]. 储能科学与技术, 2022, 11(4): 1211-1225. |
| [11] | 陶影, 赵铃飞, 王云晓, 曹余良, 侴术雷. 基于双盐高浓度电解液的高稳定性钠金属负极[J]. 储能科学与技术, 2022, 11(4): 1103-1109. |
| [12] | 岳博文, 佟佳欢, 刘玉文, 霍锋. 离子液体电解液的模拟计算方法及应用[J]. 储能科学与技术, 2022, 11(3): 897-911. |
| [13] | 赵志伟, 杨智, 彭章泉. 飞行时间二次离子质谱在锂基二次电池中的应用[J]. 储能科学与技术, 2022, 11(3): 781-794. |
| [14] | 郭云琪, 盛楠, 朱春宇, 饶中浩. 基于模板法制备氧化铝纤维及其石蜡复合相变材料热性能[J]. 储能科学与技术, 2022, 11(2): 511-520. |
| [15] | 朱兆武, 张旭堃, 苏慧, 张健, 王丽娜. 全钒液流电池提高电解液浓度的研究与应用现状[J]. 储能科学与技术, 2022, 11(11): 3439-3446. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
