Energy Storage Science and Technology ›› 2023, Vol. 12 ›› Issue (8): 2457-2481.doi: 10.19799/j.cnki.2095-4239.2023.0262
• Energy Storage Materials and Devices • Previous Articles Next Articles
Anhao ZUO( ), Ruqing FANG, Zhe LI(
), Ruqing FANG, Zhe LI( )
)
Received:2023-04-25
Revised:2023-06-02
Online:2023-08-05
Published:2023-08-23
Contact:
Zhe LI
E-mail:zah20@mails.tsinghua.edu.cn;zhe_li@tsinghua.edu.cn
CLC Number:
Anhao ZUO, Ruqing FANG, Zhe LI. Kinetic characterization of electrode materials for lithium-ion batteries via single-particle microelectrodes[J]. Energy Storage Science and Technology, 2023, 12(8): 2457-2481.

Fig. 7
(a) CV curves of LMO single particles and SEM image of particles[63]; (b) Current response and diffusion coefficient distribution of LTO thin film electrode at the constant potential[67]; (c) CV curves of LFP particles and conductive agent on the surface of composite electrode[65]; (d) Local CV curves of ZrO2-coated LCO electrode[70]"
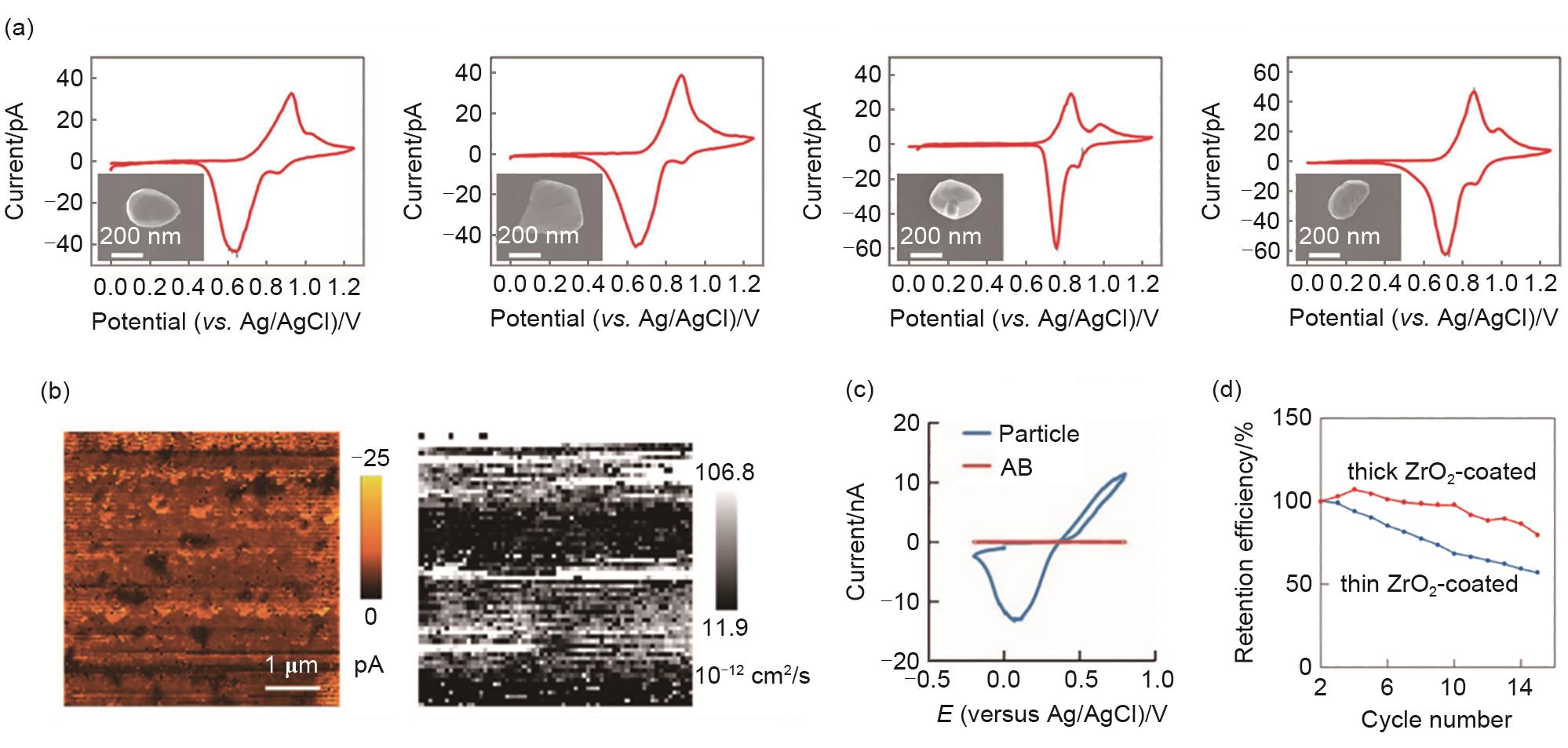

Fig. 8
(a) Surface morphology and current response of LFP composite electrode[65]; (b) Galvanostatic charging and discharging curves of a LFP single secondary particle[65]; Galvanostatic charging and discharging curves of (c) LFP single particles and (d) coin cell[68]; (e) Galvanostatic charging and discharging curves of a LMO nanoscale single particle[63]"
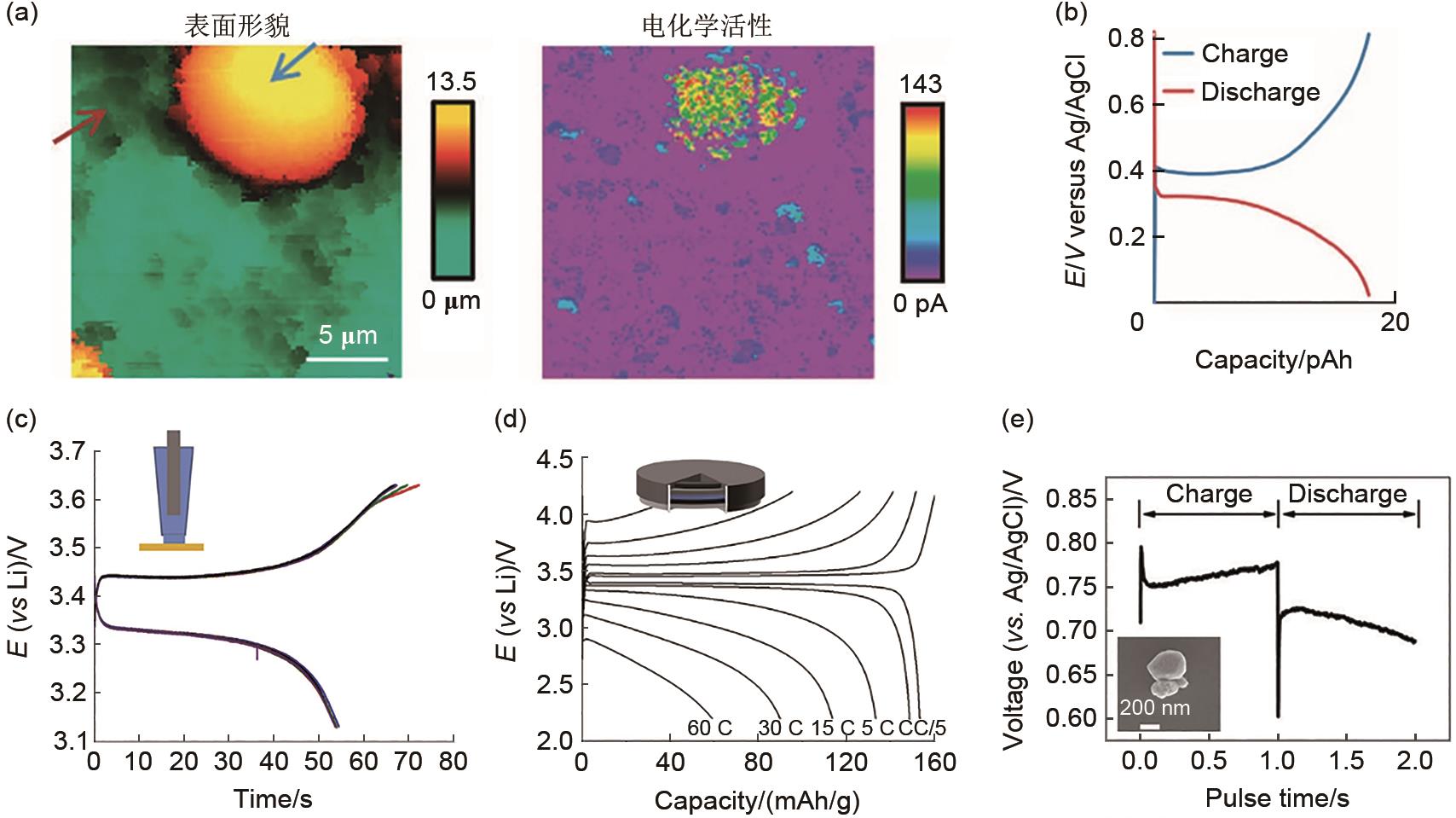

Fig. 9
(a) SEM image of nitrogen-doped reduced graphene oxide (N-rGO)/indium tin oxide (ITO) electrode surface[61]; (b) Double-layer capacitance distribution on the sample surface[61]; (c) Charge transfer resistance distribution on the sample surface[61]; Tafel curves, exchange current density, and charge transfer resistance in organic electrolyte (d) and aqueous electrolyte (e) [62]; (f) Schematic of the de-solvation process in organic and aqueous electrolyte[62]"

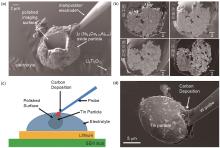
Fig. 12
(a) A microreactor with a integrated single-particle microelectrode operating in a FIB/SEM chamber[101]; (b) Evolution of the microstructure of an NCA single particle during cycling[101]; (c) Schematic of a microreactor used for in-situ observation of particle microstructure evolution and volume changes[102]; (d) SEM image of a tin single-particle microelectrode[102]"


Fig. 13
(a) The fabrication process of integrated single-particle microelectrodes[106]; (b) Electrochemical testing system of a LTO single-particle microelectrode[108]; (c) Rate performance of LTO single-particle microelectrodes at different temperatures[108]; (d) Capacity retention of LTO single-particle microelectrodes during cycling[108]; (e) Electrochemical testing system of a SiO x single-particle microelectrode[111]"


Fig. 16
(a) The fabricating process of pit-style single-particle microelectrodes[116]; (b) Schematic of the specially designed cell[116]; (c) SEM image of the pit-style single-particle microelectrode[116]; (d) Schematic of the ion-blocking electrode and its equivalent circuit model[116]; (e) Relationship between resistance, capacitance, and particle size fitted based on the equivalent circuit model[116]"
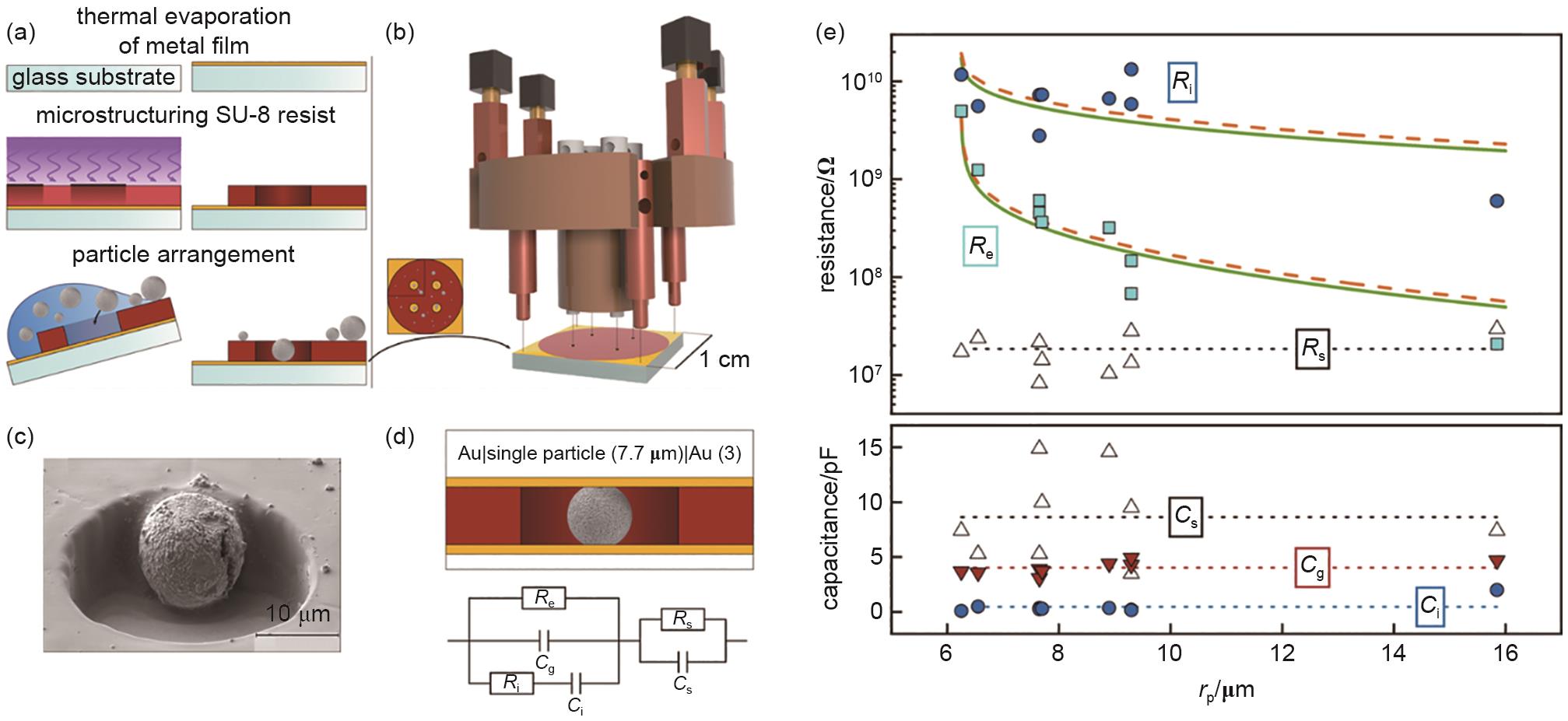
Table 1
Comparison of the above techniques for single particle measurments"
| 测试方法 | 测试体系 | 测试对象 | 对象可选择性 | 嵌锂态控制 | 受压情况 | 电化学方法的适用性 |
|---|---|---|---|---|---|---|
| 扫描电化学池显微镜 | 开放 | 单颗粒/颗粒集合 | 能 | 能 | 无 | 以CV、脉冲电流或恒电势法为主,也适用EIS |
| 接触式单颗粒微电极 | 开放 | 单颗粒 | 能 | 能 | 有 | 恒电流充放电、恒电压充放电、阻抗谱测试、倍率性能测试、循环性能测试等 |
| 连接式单颗粒微电极 (FIB/SEM内部) | 真空 | 单颗粒 | 能 | 能 | 可控 | |
| 连接式单颗粒微电极 | 封闭 | 单颗粒 | 能 | 能 | 无 | |
| 夹持式单颗粒微电极 | 封闭 | 单颗粒 | 能 | 能 | 有 | |
| 凹坑式单颗粒微电极 | 封闭 | 单颗粒 | 能 | 有潜力 | 有 | 阻抗谱 |
| 纳米碰撞法 | 封闭 | 颗粒集合 | 否 | 否 | 无 | 恒电势法 |
Table 2
Electrochemical methods for obtaining kinetic parameters of single-particles"
| 参数 | 方法 | 方法描述 | 文献 |
|---|---|---|---|
| 交换电流密度/ 电荷转移阻抗 | Tafel曲线 | 当电荷转移过程是速控步骤时,拟合Tafel方程得到交换电流密度 | [ |
| PITT | 基于考虑界面有限反应速率的电化学模型 | [ | |
| EIS | 拟合等效电路模型或阻抗谱物理模型 | [ | |
| 固相锂离子扩散系数 | 极化曲线 | 当固相扩散过程为速控步骤时,根据公式 | [ |
| GITT | 建立电化学模型,并结合恒电流边界条件得到扩散系数与电压响应的关系,据此拟合得到扩散系数 | [ | |
| PITT | 建立电化学模型,并结合恒电压边界条件得到扩散系数与电流响应的关系,据此拟合得到扩散系数 | [ | |
| EIS | 拟合Warburg模型或阻抗谱物理模型 | [ |
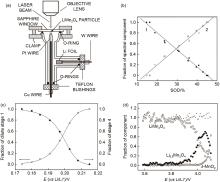
Fig. 23
(a) Schematic of a spectroelectrochemical cell for in situ Raman scattering spectroscopy of single particle microelectrodes[144]; Evolution of phase fractions during charge and discharge of single LMO polycrystalline particles (b)[144], single graphite flakes (c)[145], and single LMO crystalline particles (d)[146]"

| 1 | SCHLEUSSNER C F, ROGELJ J, SCHAEFFER M, et al. Science and policy characteristics of the Paris Agreement temperature goal[J]. Nature Climate Change, 2016, 6(9): 827-835. |
| 2 | XU G Y, SCHWARZ P, YANG H L. Adjusting energy consumption structure to achieve China's CO2 emissions peak[J]. Renewable and Sustainable Energy Reviews, 2020, 122: doi: 10.1016/j.rser. 2020.109737. |
| 3 | ZHANG H, FAN Q H, LIU S, et al. Hierarchical energy management strategy for plug-in hybrid electric powertrain integrated with dual-mode combustion engine[J]. Applied Energy, 2021, 304: doi: 10.1016/j.apenergy.2021.117869. |
| 4 | LI Z, ZUO A H, MO Z B, et al. Demonstrating stability within parallel connection as a basis for building large-scale battery systems[J]. Cell Reports Physical Science, 2022, 3(12): doi: 10. 1016/j.xcrp.2022.101154. |
| 5 | LAWDER M T, SUTHAR B, NORTHROP P W C, et al. Battery energy storage system (BESS) and battery management system (BMS) for grid-scale applications[J]. Proceedings of the IEEE, 2014, 102(6): 1014-1030. |
| 6 | LI W D, ERICKSON E M, MANTHIRAM A. High-nickel layered oxide cathodes for lithium-based automotive batteries[J]. Nature Energy, 2020, 5(1): 26-34. |
| 7 | DONG Y H, LI J. Oxide cathodes: Functions, instabilities, self healing, and degradation mitigations[J]. Chemical Reviews, 2023, 123(2): 811-833. |
| 8 | HUO H Y, JANEK J. Silicon as emerging anode in solid-state batteries[J]. ACS Energy Letters, 2022, 7(11): 4005-4016. |
| 9 | LIU X, XU G L, KOLLURU V S C, et al. Origin and regulation of oxygen redox instability in high-voltage battery cathodes[J]. Nature Energy, 2022, 7(9): 808-817. |
| 10 | SUN Y K, MYUNG S T, PARK B C, et al. High-energy cathode material for long-life and safe lithium batteries[J]. Nature Materials, 2009, 8(4): 320-324. |
| 11 | PARK G T, NAMKOONG B, KIM S B, et al. Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries[J]. Nature Energy, 2022, 7(10): 946-954. |
| 12 | CAI M Z, DONG Y H, XIE M, et al. Stalling oxygen evolution in high-voltage cathodes by lanthurization[J]. Nature Energy, 2023, 8(2): 159-168. |
| 13 | KUANG Y D, CHEN C J, KIRSCH D, et al. Thick electrode batteries: Principles, opportunities, and challenges[J]. Advanced Energy Materials, 2019, 9(33): doi: 10.1002/aenm.201901457. |
| 14 | 左安昊, 方儒卿, 李哲. 锂离子电池电极结构参数对单体能量与功率的影响[J]. 储能科学与技术, 2021, 10(2): 470-482. |
| ZUO A H, FANG R Q, LI Z. Influence of electrode structure parameters on monomer energy and power of lithium ion battery[J]. Energy Storage Science and Technology, 2021, 10(2): 470-482. | |
| 15 | ZHAO R, LIU J, GU J J. The effects of electrode thickness on the electrochemical and thermal characteristics of lithium ion battery[J]. Applied Energy, 2015, 139: 220-229. |
| 16 | DOYLE M, FULLER T F, NEWMAN J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell[J]. Journal of the Electrochemical Society, 1993, 140(6): 1526-1533. |
| 17 | FANG R Q, GE H, WANG Z H, et al. A two-dimensional heterogeneous model of lithium-ion battery and application on designing electrode with non-uniform porosity[J]. Journal of the Electrochemical Society, 2020, 167(13): doi: 10.1149/1945-7111/abb83a. |
| 18 | KUO J J, KANG S D, CHUEH W C. Contact resistance of carbon-Li x (Ni, Mn, co)O2 interfaces[J]. Advanced Energy Materials, 2022, 12(31): doi: 10.1002/aenm.202201114. |
| 19 | HEUBNER C, NICKOL A, SEEBA J, et al. Understanding thickness and porosity effects on the electrochemical performance of LiNi0.6Co0.2Mn0.2O2-based cathodes for high energy Li-ion batteries[J]. Journal of Power Sources, 2019, 419: 119-126. |
| 20 | HEUBNER C, SCHNEIDER M, MICHAELIS A. Diffusion-limited C-rate: A fundamental principle quantifying the intrinsic limits of Li-ion batteries[J]. Advanced Energy Materials, 2020, 10(2): doi: 10.1002/aenm.201902523. |
| 21 | WU J, ZHANG X, JU Z, et al. From fundamental understanding to engineering design of high-performance thick electrodes for scalable energy-storage systems[J]. Advanced Materials, 2021, 33(26): doi: 10.1002/adma.202101275. |
| 22 | DOKKO K, NAKATA N, KANAMURA K. High rate discharge capability of single particle electrode of LiCoO2[J]. Journal of Power Sources, 2009, 189(1): 783-785. |
| 23 | ZUO A H, FANG R Q, WU Z X, et al. Diffusion-limited C-rate: A criterion of rate performance for lithium-ion batteries[J]. Journal of Energy Storage, 2022, 56: doi: 10.1016/j.est.2022.105920. |
| 24 | MA Y T, LI L, QIAN J, et al. Materials and structure engineering by magnetron sputtering for advanced lithium batteries[J]. Energy Storage Materials, 2021, 39: 203-224. |
| 25 | RHO Y H, KANAMURA K. Li+ ion diffusion in Li4Ti5O12 thin film electrode prepared by PVP sol-gel method[J]. Journal of Solid State Chemistry, 2004, 177(6): 2094-2100. |
| 26 | YOON Y, PARK C, KIM J, et al. Lattice orientation control of lithium cobalt oxide cathode film for all-solid-state thin film batteries[J]. Journal of Power Sources, 2013, 226: 186-190. |
| 27 | BOHNE L, PIRK T, JAEGERMANN W. Investigations on the influence of the substrate on the crystal structure of sputtered LiCoO2[J]. Journal of Solid State Electrochemistry, 2013, 17(8): 2095-2099. |
| 28 | KANAMURA K, YAMADA Y, ANNAKA K, et al. Electrochemical evaluation of active materials for lithium ion batteries by one (single) particle measurement[J]. Electrochemistry, 2016, 84(10): 759-765. |
| 29 | CHA C S, LI C M, YANG H X, et al. Powder microelectrodes[J]. Journal of Electroanalytical Chemistry, 1994, 368(1/2): 47-54. |
| 30 | GRUET D, DELOBEL B, SICSIC D, et al. Electrochemical behavior of pure graphite studied with a powder microelectrode[J]. Electrochemistry Communications, 2018, 95: 23-27. |
| 31 | COME J, TABERNA P L, HAMELET S, et al. Electrochemical kinetic study of LiFePO4 using cavity microelectrode[J]. Journal of the Electrochemical Society, 2011, 158(10): doi: 10.1149/1.3619791. |
| 32 | ZHAO X F, ZHANG D, REN X M, et al. Quasi-random assembled single particle electrode and instinct electrochemical performance of LiFePO4 in wide temperature scope[J]. Journal of Electroanalytical Chemistry, 2014, 712: 113-118. |
| 33 | YAN K, CHEN S, MENG F W, et al. Application of ultramicroelectrode/microelectrode in fields of energy materials[J]. Chinese Science Bulletin, 2014, 59(28/29): 2851-2860. |
| 34 | YU X Q, WANG Q, ZHOU Y N, et al. High rate delithiation behaviour of LiFePO4 studied by quick X-ray absorption spectroscopy[J]. Chemical Communications, 2012, 48(94): 11537-11539. |
| 35 | FAWDON J, IHLI J, LA MANTIA F, et al. Characterising lithium-ion electrolytes via operando Raman microspectroscopy[J]. Nature Communications, 2021, 12: doi: 10.1038/s41467-021-24297-0. |
| 36 | MORIMOTO T, NAGAI M, MINOWA Y, et al. Microscopic ion migration in solid electrolytes revealed by terahertz time-domain spectroscopy[J]. Nature Communications, 2019, 10: 2662. |
| 37 | MERRYWEATHER A J, SCHNEDERMANN C, JACQUET Q, et al. Operando optical tracking of single-particle ion dynamics in batteries[J]. Nature, 2021, 594(7864): 522-528. |
| 38 | NOLAN A M, ZHU Y Z, HE X F, et al. Computation-accelerated design of materials and interfaces for all-solid-state lithium-ion batteries[J]. Joule, 2018, 2(10): 2016-2046. |
| 39 | LOMBARDO T, DUQUESNOY M, EI-BOUYSIDY H, et al. Artificial Intelligence Applied to Battery Research: Hype or Reality?[J]. Chemical Reviews, 2021: doi: 10.1021/acs.chemrev.1c00108. |
| 40 | ZHANG R, WANG C Y, GE M Y, et al. Accelerated degradation in a quasi-single-crystalline layered oxide cathode for lithium-ion batteries caused by residual grain boundaries[J]. Nano Letters, 2022, 22(9): 3818-3824. |
| 41 | KUCERNAK A. Single particle deposition on nanometer electrodes[M]//Handbook of Electrochemistry. Amsterdam: Elsevier, 2007: 709-718. |
| 42 | CLAUSMEYER J, MASA J, VENTOSA E, et al. Nanoelectrodes reveal the electrochemistry of single nickelhydroxide nanoparticles[J]. Chemical Communications, 2016, 52(11): 2408-2411. |
| 43 | REN Y Y, LIU L M, WANG Z, et al. Fabrication of single-particle microelectrodes and their electrochemical properties[J]. ACS Applied Materials & Interfaces, 2022, 14(18): 20981-20987. |
| 44 | BARD A J, FAN F R F, KWAK J, et al. Scanning electrochemical microscopy. introduction and principles[J]. Analytical Chemistry, 1989, 61(2): 132-138. |
| 45 | KWAK J, BARD A J. Scanning electrochemical microscopy. Theory of the feedback mode[J]. Analytical Chemistry, 1989, 61(11): 1221-1227. |
| 46 | KWAK J, BARD A J. Scanning electrochemical microscopy. apparatus and two-dimensional scans of conductive and insulating substrates[J]. Analytical Chemistry, 1989, 61(17): 1794-1799. |
| 47 | SANTANA J J, IZQUIERDO J, SOUTO R M. Uses of scanning electrochemical microscopy (SECM) for the characterization with spatial and chemical resolution of thin surface layers and coating systems applied on metals: A review[J]. Coatings, 2022, 12(5): 637. |
| 48 | XIA D H, WANG J Q, WU Z, et al. Sensing corrosion within an artificial defect in organic coating using SECM[J]. Sensors and Actuators B: Chemical, 2019, 280: 235-242. |
| 49 | PREET A, LIN T E. A review: Scanning electrochemical microscopy (SECM) for visualizing the real-time local catalytic activity[J]. Catalysts, 2021, 11(5): 594. |
| 50 | RYU C H, NAM Y, AHN H S. Modern applications of scanning electrochemical microscopy in the analysis of electrocatalytic surface reactions[J]. Chinese Journal of Catalysis, 2022, 43(1): 59-70. |
| 51 | BONDARENKO A, LIN T E, STUPAR P, et al. Fixation and permeabilization approaches for scanning electrochemical microscopy of living cells[J]. Analytical Chemistry, 2016, 88(23): 11436-11443. |
| 52 | FILICE F P, DING Z F. Analysing single live cells by scanning electrochemical microscopy[J]. Analyst, 2019, 144(3): 738-752. |
| 53 | BÜLTER H, PETERS F, SCHWENZEL J, et al. Spatiotemporal changes of the solid electrolyte interphase in lithium-ion batteries detected by scanning electrochemical microscopy[J]. Angewandte Chemie International Edition, 2014, 53(39): 10531-10535. |
| 54 | KUMATANI A, MATSUE T. Recent advances in scanning electrochemical microscopic analysis and visualization on lithium-ion battery electrodes[J]. Current Opinion in Electrochemistry, 2020, 22: 228-233. |
| 55 | 贾铮, 戴长松, 陈玲. 电化学测量方法[M]. 北京: 化学工业出版社, 2006. |
| JIA Z, DAI C S, CHEN L. Electrochemical measurement method[M]. Beijing: Chemical Industry Press, 2006. | |
| 56 | EBEJER N, GÜELL A G, LAI S C S, et al. Scanning electrochemical cell microscopy: A versatile technique for nanoscale electrochemistry and functional imaging[J]. Annual Review of Analytical Chemistry, 2013, 6: 329-351. |
| 57 | EBEJER N, SCHNIPPERING M, COLBURN A W, et al. Localized high resolution electrochemistry and multifunctional imaging: Scanning electrochemical cell microscopy[J]. Analytical Chemistry, 2010, 82(22): 9141-9145. |
| 58 | TAKAHASHI Y, MARINA M, ANDO T, et al. Electrochemical image of BDD[M]//EINAGA Y. Diamond Electrodes. Singapore: Springer, 2022: 43-55. |
| 59 | BARKER A L, GONSALVES M, MACPHERSON J V, et al. Scanning electrochemical microscopy: Beyond the solid/liquid interface[J]. Analytica Chimica Acta, 1999, 385(1/2/3): 223-240. |
| 60 | TAO B L, MCPHERSON I J, DAVIDDI E, et al. Multiscale electrochemistry of lithium manganese oxide (LiMn2O4): From single particles to ensembles and degrees of electrolyte wetting[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(4): 1459-1471. |
| 61 | CHENG L, JIN R, JIANG D C, et al. Scanning electrochemical cell microscopy platform with local electrochemical impedance spectroscopy[J]. Analytical Chemistry, 2021, 93(49): 16401-16408. |
| 62 | YAMAMOTO T, ANDO T, KAWABE Y, et al. Characterization of the depth of discharge-dependent charge transfer resistance of a single LiFePO4 particle[J]. Analytical Chemistry, 2021, 93(43): 14448-14453. |
| 63 | TAO B L, YULE L C, DAVIDDI E, et al. Correlative electrochemical microscopy of Li-ion (de) intercalation at a series of individual LiMn2O4 particles[J]. Angewandte Chemie, 2019, 131(14): 4654-4659. |
| 64 | KUMATANI A, TAKAHASHI Y, MIURA C, et al. Scanning electrochemical cell microscopy for visualization and local electrochemical activities of lithium-ion (de) intercalation process in lithium-ion batteries electrodes[J]. Surface and Interface Analysis, 2019, 51(1): 27-30. |
| 65 | TAKAHASHI Y, KUMATANI A, MUNAKATA H, et al. Nanoscale visualization of redox activity at lithium-ion battery cathodes[J]. Nature Communications, 2014, 5: 5450. |
| 66 | MARTÍN-YERGA D, KANG M, UNWIN P R. Scanning electrochemical cell microscopy in a glovebox: Structure-activity correlations in the early stages of solid-electrolyte interphase formation on graphite[J]. ChemElectroChem, 2021, 8(22): 4240-4251. |
| 67 | TAKAHASHI Y, YAMASHITA T, TAKAMATSU D, et al. Nanoscale kinetic imaging of lithium ion secondary battery materials using scanning electrochemical cell microscopy[J]. Chemical Communications, 2020, 56(65): 9324-9327. |
| 68 | SNOWDEN M E, DAYEH M, PAYNE N A, et al. Measurement on isolated lithium iron phosphate particles reveals heterogeneity in material properties distribution[J]. Journal of Power Sources, 2016, 325: 682-689. |
| 69 | DAYEH M, ZAMANZAD GHAVIDEL D M R, MAUZEROLL D J, et al. Micropipette contact method to investigate high-energy cathode materials by using an ionic liquid[J]. ChemElectroChem, 2019, 6(1): 195-201. |
| 70 | INOMATA H, TAKAHASHI Y, TAKAMATSU D, et al. Visualization of inhomogeneous current distribution on ZrO2-coated LiCoO2 thin-film electrodes using scanning electrochemical cell microscopy[J]. Chemical Communications, 2019, 55(4): 545-548. |
| 71 | BENTLEY C L, KANG M, UNWIN P R. Nanoscale structure dynamics within electrocatalytic materials[J]. Journal of the American Chemical Society, 2017, 139(46): 16813-16821. |
| 72 | BENTLEY C L, PERRY D, UNWIN P R. Stability and placement of Ag/AgCl quasi-reference counter electrodes in confined electrochemical cells[J]. Analytical Chemistry, 2018, 90(12): 7700-7707. |
| 73 | WAHAB O J, KANG M, UNWIN P R. Scanning electrochemical cell microscopy: A natural technique for single entity electrochemistry[J]. Current Opinion in Electrochemistry, 2020, 22: 120-128. |
| 74 | BENTLEY C L, EDMONDSON J, MELONI G N, et al. Nanoscale electrochemical mapping[J]. Analytical Chemistry, 2019, 91(1): 84-108. |
| 75 | BENTLEY C L, KANG M, UNWIN P R. Scanning electrochemical cell microscopy: New perspectives on electrode processes in action[J]. Current Opinion in Electrochemistry, 2017, 6(1): 23-30. |
| 76 | BURSELL M, BJÖRNBOM P. A method for studying microelectrodes by means of micromanipulators as applied to carbon agglomerates from oxygen reduction electrode catalyst[J]. Journal of the Electrochemical Society, 1990, 137(1): 363-364. |
| 77 | UCHIDA I, FUJIYOSHI H, WAKI S. Microvoltammetric studies on single particles of battery active materials[J]. Journal of Power Sources, 1997, 68(1): 139-144. |
| 78 | WAKI S, DOKKO K, MATSUE T, et al. Microvoltammetric studies on single particle voltammetry of LiNiO2 and LiCoO2. In situ observation of particle splitting during Li-ion extraction/insertion[J]. Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 1997, 65(11): 954-962. |
| 79 | DOKKO K, HORIKOSHI S, ITOH T, et al. Microvoltammetry for cathode materials at elevated temperatures: Electrochemical stability of single particles[J]. Journal of Power Sources, 2000, 90(1): 109-115. |
| 80 | DOKKO K, MOHAMEDI M, UMEDA M, et al. Kinetic study of Li-ion extraction and insertion at LiMn2O4 single particle electrodes using potential step and impedance methods[J]. Journal of the Electrochemical Society, 2003, 150(4): doi: 10.1149/1.1556596. |
| 81 | DOKKO K, NISHIZAWA M, UCHIDA I. High resolution cyclic voltammograms of LiMn2- xNixO4 with a microelectrode technique[J]. Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 1998, 66(12): 1188-1193. |
| 82 | MUNAKATA H, TAKEMURA B, SAITO T, et al. Evaluation of real performance of LiFePO4 by using single particle technique[J]. Journal of Power Sources, 2012, 217: 444-448. |
| 83 | ANDO K, YAMADA Y, NISHIKAWA K, et al. Degradation analysis of LiNi0.8Co0.15Al0.05O2 for cathode material of lithium-ion battery using single-particle measurement[J]. ACS Applied Energy Materials, 2018, 1(9): 4536-4544. |
| 84 | KIM J S, LIM S, MUNAKATA H, et al. Understanding the relationship of electrochemical properties and structure of microstructure-controlled core shell gradient type Ni-rich cathode material by single particle measurement[J]. Electrochimica Acta, 2021, 390: doi: 10.1016/j.electacta.2021.138813. |
| 85 | WAKI S, DOKKO K, ITOH T, et al. High-Speed voltammetry of Mn-doped LiCoO2 using a microelectrode technique[J]. Journal of Solid State Electrochemistry, 2000, 4(4): 205-209. |
| 86 | TAKAHASHI Y, KIJIMA N, DOKKO K, et al. Structure and electron density analysis of electrochemically and chemically delithiated LiCoO2 single crystals[J]. Journal of Solid State Chemistry, 2007, 180(1): 313-321. |
| 87 | DOKKO K, NISHIZAWA M, MOHAMEDI M, et al. Electrochemical studies of Li-ion extraction and insertion of LiMn2O4 single crystal[J]. Electrochemical and Solid-State Letters, 2001, 4(9): doi: 10.1149/1.1389875. |
| 88 | NISHIZAWA M. Measurements of chemical diffusion coefficient of lithium ion in graphitized mesocarbon microbeads using a microelectrode[J]. Electrochemical and Solid-State Letters, 1999, 1(1): 10. |
| 89 | UMEDA M, DOKKO K, FUJITA Y, et al. Electrochemical impedance study of Li-ion insertion into mesocarbon microbead single particle electrode[J]. Electrochimica Acta, 2001, 47(6): 885-890. |
| 90 | DOKKO K, NAKATA N, SUZUKI Y, et al. High-rate lithium deintercalation from lithiated graphite single-particle electrode[J]. The Journal of Physical Chemistry C, 2010, 114(18): 8646-8650. |
| 91 | DOKKO K, FUJITA Y, MOHAMEDI M, et al. Electrochemical impedance study of Li-ion insertion into mesocarbon microbead single particle electrode[J]. Electrochimica Acta, 2001, 47(6): 933-938. |
| 92 | DOKKO K, YOSHIDA K, NOZAWA R, et al. Limitation of rate capability of Li4Ti5O12 single-particle[J]. ECS Meeting Abstracts, 2012, (10): 994. |
| 93 | FUKUI H, NAKATA N, DOKKO K, et al. Lithiation and delithiation of silicon oxycarbide single particles with a unique microstructure[J]. ACS Applied Materials & Interfaces, 2011, 3(7): 2318-2322. |
| 94 | 王福庆, 魏奕民, 苏育专, 等. LiFePO4单颗粒电化学本征性能的快速精确评测[J]. 电化学, 2015, 21(6): 566-571. |
| WANG F Q, WEI Y M, SU Y Z, et al. Fast and accurate evaluation of LiFePO4 cathode materials by single particle microelectrode[J]. Journal of Electrochemistry, 2015, 21(6): 566-571. | |
| 95 | 魏奕民. 高镍三元正极材料动力学性能的单颗粒研究[J]. 电化学, 2018, 24(1): 81-88. |
| WEI Y M. Intrinsic kinetic properties of ternary material for lithium ion batteries assessed by single particle microelectrode[J]. Journal of Electrochemistry, 2018, 24(1): 81-88. | |
| 96 | HUANG Y H, WANG F M, HUANG T T, et al. Micro-electrode linked cyclic voltammetry study reveals ultra-fast discharge and high ionic transfer behavior of LiFePO4[J]. International Journal of Electrochemical Science, 2012, 7(2): 1205-1213. |
| 97 | DOKKO K, MOHAMEDI M, FUJITA Y, et al. Kinetic characterization of single particles of LiCoO2 by AC impedance and potential step methods[J]. Journal of the Electrochemical Society, 2001, 148(5): doi: 10.1149/1.1359197. |
| 98 | KOZAWA T, NISHIKAWA K. Macroporous Mn3O4 microspheres as a conversion-type anode material morphology for Li-ion batteries[J]. Journal of Solid State Electrochemistry, 2020, 24(6): 1283-1290. |
| 99 | DOKKO K. In situ observation of LiNiO2 single-particle fracture during Li-ion extraction and insertion[J]. Electrochemical and Solid-State Letters, 1999, 3(3): 125. |
| 100 | NISHIZAWA M, UCHIDA I. Microelectrode-based characterization systems for advanced materials in battery and sensor applications[J]. Electrochimica Acta, 1999, 44(21/22): 3629-3637. |
| 101 | MILLER D J, PROFF C, WEN J G, et al. Observation of microstructural evolution in Li battery cathode oxide particles by in situ electron microscopy[J]. Advanced Energy Materials, 2013, 3(8): 1098-1103. |
| 102 | ZHOU X W, LI T Y, CUI Y, et al. In situ focused ion beam scanning electron microscope study of microstructural evolution of single tin particle anode for Li-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(2): 1733-1738. |
| 103 | ZHOU X W, LI T Y, CUI Y, et al. In situ focused ion beam-scanning electron microscope study of crack and nanopore formation in germanium particle during (de) lithiation[J]. ACS Applied Energy Materials, 2019, 2(4): 2441-2446. |
| 104 | ZHOU X W, LI T Y, CUI Y, et al. In situ and operando morphology study of germanium-selenium alloy anode for lithium-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(7): 6115-6120. |
| 105 | LU W Q, ZHOU X W, LIU Y Z, et al. Crack-free silicon monoxide as anodes for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(51): 57141-57145. |
| 106 | SAKURAI Y, KAWASHIRI S, SUETOME H, et al. Single particle measurement of battery electrode materials by the particle/current collector integrated microelectrode[Z]. 65th Annual Meeting of the International Society of Electrochemistry, 2014 |
| 107 | SAKURAI Y, KAWASHIRI S, UTAGAWA M, et al. Electrochemical characterization of Li4Ti5O12 by single particle measurements using a particle-current collector integrated microelectrode[C]. The Electrochemical Societ, 2015 |
| 108 | TOJO T, KAWASHIRI S, TSUDA T, et al. Electrochemical performance of single Li4Ti5O12 particle for lithium ion battery anode[J]. Journal of Electroanalytical Chemistry, 2019, 836: 24-29. |
| 109 | INADA R, KUMASAKA R, INABE S, et al. Li+ insertion/extraction properties for TiNb2O7 single particle characterized by a particle-current collector integrated microelectrode[J]. Journal of the Electrochemical Society, 2018, 166(3): doi: 10.1149/2.0241903jes. |
| 110 | FANG R Q, ZUO A H, LI Z. The fabrication of SiOx single particle electrode and its electrochemo-mechanical study[J]. Journal of Power Sources, 2022, 549: doi: 10.1016/j.jpowsour.2022.232146. |
| 111 | FANG R Q, ZUO A H, LI Z. Characterization of electrochemical kinetics of SiOx using single-particle electrode technique: An impedance study[J]. Electrochemistry Communications, 2022, 140: doi: 10.1016/j.elecom.2022.107342. |
| 112 | TSAI P C, WEN B H, WOLFMAN M, et al. Single-particle measurements of electrochemical kinetics in NMC and NCA cathodes for Li-ion batteries[J]. Energy & Environmental Science, 2018, 11(4): 860-871. |
| 113 | WEN B H, DENG Z, TSAI P C, et al. Ultrafast ion transport at a cathode-electrolyte interface and its strong dependence on salt solvation[J]. Nature Energy, 2020, 5(8): 578-586. |
| 114 | LI X, LI N, ZHANG K L, et al. Correlating electrochemical kinetic parameters of single LiNi1/3Mn1/3Co1/3O2 particles with the performance of corresponding porous electrodes[J]. Angewandte Chemie, 2022, 134(34): doi: 10.1002/ange.202205394. |
| 115 | CHEN T, WU J, ZHANG Q L, et al. Recent advancement of SiOx based anodes for lithium-ion batteries[J]. Journal of Power Sources, 2017, 363: 126-144. |
| 116 | BURKHARDT S, FRIEDRICH M S, ECKHARDT J K, et al. Charge transport in single NCM cathode active material particles for lithium-ion batteries studied under well-defined contact conditions[J]. ACS Energy Letters, 2019, 4(9): 2117-2123. |
| 117 | 李婷, 刘洋, 蒋亚楠, 等. 单颗粒电化学分析[J]. 中国科学: 化学, 2016, 46(10): 1064-1079. |
| LI T, LIU Y, JIANG Y N, et al. Electrochemical analysis of single particles[J]. Scientia Sinica Chimica, 2016, 46(10): 1064-1079. | |
| 118 | 徐为, 邹国强, 侯红帅, 等. 电池材料单颗粒分析方法[J]. 硅酸盐学报, 2022, 50(1): 185-193. |
| XU W, ZOU G Q, HOU H S, et al. Single particle analysis method for battery materials[J]. Journal of the Chinese Ceramic Society, 2022, 50(1): 185-193. | |
| 119 | ZAMPARDI G, BATCHELOR-MCAULEY C, KÄTELHÖN E, et al. Lithium-ion-transfer kinetics of single LiMn2O4 particles[J]. Angewandte Chemie International Edition, 2017, 56(2): 641-644. |
| 120 | XU W, ZHOU Y G, JI X B. Lithium-ion-transfer kinetics of single LiFePO4 particles[J]. The Journal of Physical Chemistry Letters, 2018, 9(17): 4976-4980. |
| 121 | ZHU Y J, WANG C S. Galvanostatic intermittent titration technique for phase-transformation electrodes[J]. The Journal of Physical Chemistry C, 2010, 114(6): 2830-2841. |
| 122 | XU W, ZHOU Y G, CHEN J, et al. Single LiNi0.8Mn0.1Co0.1O2 particle electrochemistry of collision[J]. Journal of Power Sources, 2021, 506: doi: 10.1016/j.jpowsour.2021.230228. |
| 123 | LÖFFLER T, CLAUSMEYER J, WILDE P, et al. Single entity electrochemistry for the elucidation of lithiation kinetics of TiO2 particles in non-aqueous batteries[J]. Nano Energy, 2019, 57: 827-834. |
| 124 | EDGAR V. Why nanoelectrochemistry is necessary in battery research?[J]. Current Opinion in Electrochemistry, 2020, 25: doi: 10.1016/j.coelec.2020.09.002. |
| 125 | ZAMPARDI G, SOKOLOV S V, BATCHELOR-MCAULEY C, et al. Potassium (De-) insertion processes in prussian blue particles: Ensemble versus single nanoparticle behaviour[J]. Chemistry-A European Journal, 2017, 23(57): 14338-14344. |
| 126 | DOKKO K, HORIKOSHI S, ITOH T, et al. Rapid evaluation of charge/discharge properties for lithium manganese oxide particles at elevated temperatures[J]. Journal of Solid State Electrochemistry, 2002, 6(3): 188-193. |
| 127 | UMIROV N, YAMADA Y, MUNAKATA H, et al. Analysis of intrinsic properties of Li4Ti5O12 using single-particle technique[J]. Journal of Electroanalytical Chemistry, 2019, 855: doi: 10.1016/j.jelechem.2019.113514. |
| 128 | SHAJU K M, SUBBA RAO G V, CHOWDARI B V R. Electrochemical kinetic studies of Li-ion in O2 -Structured Li2/3(Ni1/3Mn2/3)O2 and Li(2/3)+ x(Ni1/3Mn2/3)O2 by EIS and GITT[J]. Journal of the Electrochemical Society, 2003, 150(1): doi: 10.1149/1.1521754. |
| 129 | YANG S Y, WANG X Y, YANG X K, et al. Determination of the chemical diffusion coefficient of lithium ions in spherical Li[Ni0.5Mn0.3Co0.2]O2[J]. Electrochimica Acta, 2012, 66: 88-93. |
| 130 | BAI Y S, WANG X Y, ZHANG X Y, et al. The kinetics of Li-ion deintercalation in the Li-rich layered Li1.12[Ni0.5Co0.2Mn0.3]0.89O2 studied by electrochemical impedance spectroscopy and galvanostatic intermittent titration technique[J]. Electrochimica Acta, 2013, 109: 355-364. |
| 131 | LIM S, KIM J H, YAMADA Y, et al. Improvement of rate capability by graphite foam anode for Li secondary batteries[J]. Journal of Power Sources, 2017, 355: 164-170. |
| 132 | CHAE J E, ANNAKA K, HONG K, et al. Electrochemical characterization of phosphorous-doped soft carbon using single particle for lithium battery anode[J]. Electrochimica Acta, 2014, 130: 60-65. |
| 133 | REINOLD L M, YAMADA Y, GRACZYK-ZAJAC M, et al. The influence of the pyrolysis temperature on the electrochemical behavior of carbon-rich SiCN polymer-derived ceramics as anode materials in lithium-ion batteries[J]. Journal of Power Sources, 2015, 282: 409-415. |
| 134 | YAMADA Y, NODA Y, MUNAKATA H, et al. Investigation of carbon-coating effect on the electrochemical performance of LiCoPO4 single particle[J]. Electrochemistry, 2018, 86(3): 145-151. |
| 135 | GOTO Y, NAKANISHI S, NAKAI Y, et al. The crystal structure and electrical/thermal transport properties of Li1- xSn2+ xP2 and its performance as a Li-ion battery anode material[J]. Journal of Materials Chemistry A, 2021, 9(11): 7034-7041. |
| 136 | WANG F Q, JIANG Y, LIN S L, et al. High-voltage performance of LiCoO2 cathode studied by single particle microelectrodes-influence of surface modification with TiO2[J]. Electrochimica Acta, 2019, 295: 1017-1026. |
| 137 | NISHIZAWA M, KOSHIKA H, HASHITANI R, et al. Ion- and electron-transport properties of a single particle of disordered carbon during the lithium insertion reaction[J]. The Journal of Physical Chemistry B, 1999, 103(24): 4933-4936. |
| 138 | HUANG J, GE H, LI Z, et al. An agglomerate model for the impedance of secondary particle in lithium-ion battery electrode[J]. Journal of the Electrochemical Society, 2014, 161(8): doi: 10. 1149/2.027408jes. |
| 139 | CHURIKOV A V, IVANISHCHEV A V, IVANISHCHEVA I A, et al. Determination of lithium diffusion coefficient in LiFePO4 electrode by galvanostatic and potentiostatic intermittent titration techniques[J]. Electrochimica Acta, 2010, 55(8): 2939-2950. |
| 140 | FANG R Q, LI Z. A modeling framework of electrochemo-mechanics of lithium-ion battery: Part I. impedance response of single particle[J]. Journal of the Electrochemical Society, 2021, 168(12): doi: 10. 1149/1945-7111/ac3e4b. |
| 141 | 李文俊, 褚赓, 彭佳悦, 等. 锂离子电池基础科学问题(Ⅻ)——表征方法[J]. 储能科学与技术, 2014, 3(6): 642-667. |
| LI W J, CHU G, PENG J Y, et al. Fundamental scientific aspects of lithium batteries(Ⅻ)—Characterization techniques[J]. Energy Storage Science and Technology, 2014, 3(6): 642-667. | |
| 142 | ROMANO BRANDT L, MARIE J J, MOXHAM T, et al. Synchrotron X-ray quantitative evaluation of transient deformation and damage phenomena in a single nickel-rich cathode particle[J]. Energy & Environmental Science, 2020, 13(10): 3556-3566. |
| 143 | LI S F, JIANG Z S, HAN J X, et al. Mutual modulation between surface chemistry and bulk microstructure within secondary particles of nickel-rich layered oxides[J]. Nature Communications, 2020, 11: 4433. |
| 144 | DOKKO K, SHI Q F, STEFAN I C, et al. In situ Raman spectroscopy of single microparticle Li+-intercalation electrodes[J]. The Journal of Physical Chemistry B, 2003, 107(46): 12549-12554. |
| 145 | SHI Q F, TAKAHASHI Y, AKIMOTO J, et al. In situ Raman scattering measurements of a LiMn2O4 single crystal microelectrode[J]. Electrochemical and Solid-State Letters, 2005, 8(10): doi: 10. 1149/1.2030507. |
| 146 | SHI Q F, DOKKO K, SCHERSON D A. In situ Raman microscopy of a single graphite microflake electrode in a Li+-containing electrolyte[J]. The Journal of Physical Chemistry B, 2004, 108(15): 4789-4793. |
| [1] | Jiaxing YANG, Hengyun ZHANG, Yidong XU. Heat generation analysis for lithium-ion battery components using electrochemical and thermal coupled model [J]. Energy Storage Science and Technology, 2023, 12(8): 2615-2625. |
| [2] | Jilu ZHANG, Yuchen DONG, Qiang SONG, Siming YUAN, Xiaodong GUO. Controllable synthesis and electrochemical mechanism related to polycrystalline and single-crystalline Ni-rich layered LiNi0.9Co0.05Mn0.05O2 cathode materials [J]. Energy Storage Science and Technology, 2023, 12(8): 2382-2389. |
| [3] | Yu GUO, Yiwei WANG, Juan ZHONG, Jinqiao DU, Jie TIAN, Yan LI, Fangming JIANG. Fault diagnosis method for microinternal short circuits in lithium-ion batteries based on incremental capacity curve [J]. Energy Storage Science and Technology, 2023, 12(8): 2536-2546. |
| [4] | Qingsong ZHANG, Fangwei BAO, Jiangjao NIU. Risk analysis method of thermal runaway gas explosion in lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(7): 2263-2270. |
| [5] | Yubo ZHANG, Youyuan WANG, Dongning HUANG, Ziyi WANG, Weigen CHEN. Prognostic method of lithium-ion battery lifetime degradation under various working conditions [J]. Energy Storage Science and Technology, 2023, 12(7): 2238-2245. |
| [6] | Qinpei CHEN, Xuehui WANG, Wenzhong MI. Experiential study on the toxic and explosive characteristics of thermal runaway gas generated in electric-vehicle lithium-ion battery systems [J]. Energy Storage Science and Technology, 2023, 12(7): 2256-2262. |
| [7] | Wenda ZAN, Rui ZHANG, Fei DING. Development and application of electrochemical models for lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(7): 2302-2318. |
| [8] | Hongsheng GUAN, Cheng QIAN, Binghui XU, Bo SUN, Yi REN. SAM-GRU-based fusion neural network for SOC estimation in lithium-ion batteries under a wide range of operating conditions [J]. Energy Storage Science and Technology, 2023, 12(7): 2229-2237. |
| [9] | Maosong FAN, Mengmeng GENG, Guangjin ZHAO, Kai YANG, Fangfang WANG, Hao LIU. Research on battery sorting technology for echelon utilization based on multifrequency impedance [J]. Energy Storage Science and Technology, 2023, 12(7): 2202-2210. |
| [10] | Yi WANG, Xuebing CHEN, Yuanxi WANG, Jieyun ZHENG, Xiaosong LIU, Hong LI. Overview of multilevel failure mechanism and analysis technology of energy storage lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(7): 2079-2094. |
| [11] | Shuqin LIU, Xiaoyan WANG, Zhendong ZHANG, Zhenxia DUAN. Experimental and simulation research on liquid-cooling system of lithium-ion battery packs [J]. Energy Storage Science and Technology, 2023, 12(7): 2155-2165. |
| [12] | Yuxin CHEN, Jiamu YANG, Dongbo LI, Cheng LIAN, Honglai LIU. Numerical simulation of the vacuum drying process of cylindrical lithium-ion batteries [J]. Energy Storage Science and Technology, 2023, 12(6): 1957-1967. |
| [13] | Fang LI, Yongjun MIN, Yong ZHANG. Review of key technology research on the reliability of power lithium batteries based on big data [J]. Energy Storage Science and Technology, 2023, 12(6): 1981-1994. |
| [14] | Yongli YI, Ran YU, Wu LI, Yi JIN, Zheren DAI. Preparation of Mo, Al-doped Li7La3Zr2O12-based composite solid electrolyte and performance of all-solid-state batterys [J]. Energy Storage Science and Technology, 2023, 12(5): 1490-1499. |
| [15] | Luhao HAN, Ziyang WANG, Xiaolong HE, Chunshan HE, Xiaolong SHI, Bin YAO. The effect of water mist strategies on thermal runaway fire suppression of large-capacity NCM lithium-ion battery [J]. Energy Storage Science and Technology, 2023, 12(5): 1664-1674. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
