Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (9): 3340-3353.doi: 10.19799/j.cnki.2095-4239.2025.0157
• Energy Storage Materials and Devices • Previous Articles Next Articles
Jinfeng WANG1( ), Yue LIU2, Hongjie ZHONG1, Junming CAO2(
), Yue LIU2, Hongjie ZHONG1, Junming CAO2( ), Xinglong WU1,2(
), Xinglong WU1,2( )
)
Received:2025-02-22
Revised:2025-03-18
Online:2025-09-28
Published:2025-09-05
Contact:
Junming CAO, Xinglong WU
E-mail:wangjinfeng123@nenu.edu.cn;jmcao@nenu.edu.cn;xinglong@nenu.edu.cn
CLC Number:
Jinfeng WANG, Yue LIU, Hongjie ZHONG, Junming CAO, Xinglong WU. Recent advances in structural design, synthesis, and electrochemical applications of Mo-based electrode materials[J]. Energy Storage Science and Technology, 2025, 14(9): 3340-3353.
Table 1
Typical synthesis methods and electrochemical performance of molybdenum-based materials"
| 材料 | 合成方法 | 初始容量/电流密度 | 应用范围 |
|---|---|---|---|
| α-MoO3/SWCNH[ | 微波水热 | 654 mAh/g @ 1 C | 锂离子电池 |
| MoS2 foam[ | 电流体动力学(EHD)打印 | 1515 mAh/g @ 1 A/g | 锂离子电池 |
| 2H-MoTe2[ | 固相 | 432 mAh/g @ 1 A/g | 锂离子电池 |
| MoO2/MoS2@NC[ | 空气热活化 | 748 mAh/g @ 1 A/g | 锂离子电池 |
| MoO2/Mo2C/C[ | 碳化 | 482.5 mAh/g @ 1 A/g | 锂离子电池 |
| MoSe2/MoC/N-C[ | 退火-硒化 | 648 mAh/g @ 1 A/g | 锂离子电池 |
| N,P-rGO/h-MoO2[ | 水热 | 824.6 mAh/g/1 C | 锂硫电池 |
| Mo2C/C[ | 溶剂热-退火 | 946.7 mAh/g @ 1 C | 锂硫电池 |
| MoN@CMK-5[ | 熔融法 | 853.3 mAh/g @ 1 C | 锂硫电池 |
| DMcT-MoO3[ | 溶剂热 | 205 mAh/g @ 1 A/g | 钠离子电池 |
| N-MoS2/C@SiOC[ | 热解 | 376.1 mAh/g @ 1 A/g | 钠离子电池 |
| CoS/MoS2[ | 水热-固态硫化 | 537.4 mAh/g @ 1 A/g | 钠离子电池 |
| MoTe2(Blocks)[ | 固相 | 320 mAh/g @ 1 A/g | 钠离子电池 |
| Meso-Mo3N2-NWs[ | 共沉淀法 | 272 mAh/g @ 1 A/g | 钠离子电池 |
| MoO3-MoS2[ | 水热-气相沉积 | 401 mAh/g @ 1 A/g | 钠离子电池 |
| MoSe2 HNRAs[ | 沉积-硒化 | 330 mAh/g @ 1 A/g | 铝离子电池 |
| MoP@NPCNFs[ | 静电纺丝 | 230 mAh/g @ 1 A/g | 钾离子电池 |
| MoO2@NC[ | 还原-退火 | 103 mAh/g @ 1 A/g | 锌离子电池 |
| MoSe2-VSe[ | 溶剂热-退火 | 50.8 mAh/g @ 1 A/g | 锌离子电池 |
| MoS2/PGF[ | 微流控法 | 633 mF/cm2 @ 1 mA/cm2 | 超级电容器 |
| NF@MnMoO4[ | 水热 | 4609 F/g @ 1 A/g | 超级电容器 |
| P-MnMoO4[ | 水热-气-固反应 | 2.112 F/cm2 @ 1 mA/cm2 | 超级电容器 |
| CoMoO4@CoP/BGA[ | 溶剂热 | 3056.4 F/g @ 1 A/g | 超级电容器 |
| Ni/Ni3S2@NiMoO4[ | 水热 | 1327.3 µAh/cm2 @ 2 mA/cm2 | 超级电容器 |
| NiCo2O4@NiMoO4[ | 水热-退火 | 2.522F cm-2 @ 1 mA/cm2 | 超级电容器 |
| MoS2/MoO3@graphite[ | 微波固相法 | 268.5 F/g @ 1 A/g | 超级电容器 |
| MoSe2-Mo2C[ | 水热 | 850 F/g @ 1 A/g | 超级电容器 |
| Mo2C/Mo2N[ | 水热-退火 | 2050.2 F/g @ 1 A/g | 超级电容器 |
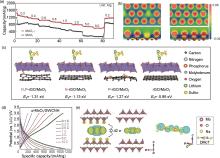
Fig. 2
(a) Rate behavior of discharge-charge capacities at different current densities of MoO2-δ and MoO2; (b) The top (upper panel) and side (lower panel) views of charge density difference for MoO2-δ and MoO2[19]; (c) DFT calculation of N, P-rGO/MoO2 with heteroatom variation[20]; (d) Galvanostatic charge-discharge profile of α-MoO3/SWCNH composite at different C-rates[23]; (e) Charge density difference of MoO3 intercalated by molecules[24]"
Fig. 3
(a) The structural evolution of the MoS2 foam; (b) The cyclic voltammograms of architected MoS2 foam anode at a scan rate of 0.3 mV/s[34]; (c) Schematic of major roles of the nanoscale SiOC shell in the N-MoS2/C@SiOC composite during the sodium-ion storage process[35]; (d) The plot of log (peak current) versus log (scan rate) at each peak on the CV curves of MoSe2 and MoSe2-VSe; (e) Calculated ground and excited Gibbs free energy of reactive stages[38]; (f) Cycling performance with Coulombic efficiency during 200 cycles at 1 A/g current rate[43]; (g) Schematic of reversible Li storage mechanism in MoTe2[44]"
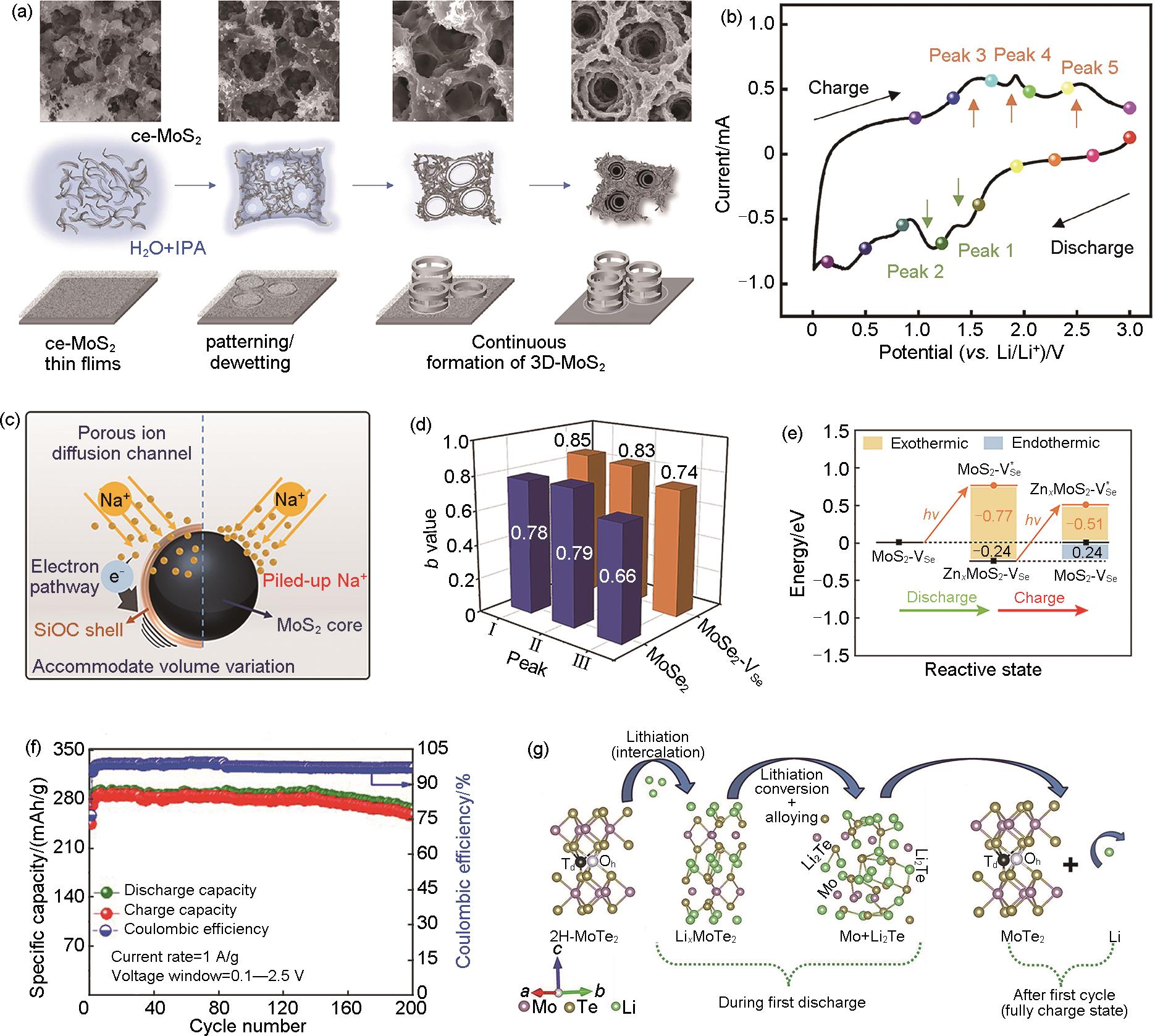
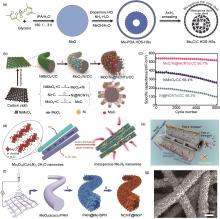
Fig. 4
(a) Schematic illustration of the synthesis of Mo2C/C HDS-HSs[47]; (b) Illustration of the formation and structure of MoC/Ni@NCNTs/CC; (c) Cycling life test of NiMoO4/CC, Ni@NCNTs/CC and MoC/Ni@NCNTs/CC[49]; (d) Schematic of the transformation from Mo3O10(C6H8N)2-2H2O nanowires to Meso-Mo3N2-NWs; (e) Electrochemical reaction mechanism diagram of MoN@CMK-5/S[51]; (f) Schematic diagram of the preparation procedure of NCNF@MoP; (g) FESEM images of NCNF@MoP[54]"
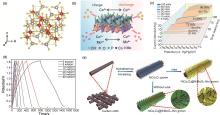
Fig. 5
(a) Crystal structure of MnMoO4[57]; (b) Schematic diagram of the pseudocapacitive reaction mechanism in CoMoO4@CoP/BGA; (c) Surface capacitive contribution ratio at different scan rates[59]; (d) Charge and discharge of Ni/Ni3S2@NiMoO4//AC cells at different current densities[60]; (e) Schematic fabrication processes of NiCo2O4@NiMoO4/CC with different shell thickness[61]"
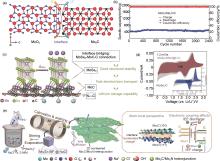
Fig. 6
(a) DOS of the MoO2/Mo2C heterostructure; (b) MoO2/Mo2C/C cycling properties at 1 A/g[68]; (c) Model of interface bridging between MoSe2- and N-doped carbon network; (d) Capacitive contribution and diffusion contribution at 1.2 mV/s[69]; (e) Schematic illustration for the synthesis strategy of 2D nonlayered Mo2C/Mo2N heterojunction nanosheets assembled by interconnected Mo2C and Mo2N nanocrystals, along with the theoretical model of Mo2C/Mo2N heterojunction and the analysis of electron interaction on heterointerface[71]"
| [1] | RONG Y, SANG J, CHE L, et al. Designing electrolytes for aqueous electrocatalytic CO2 reduction[J]. Acta Physico Chimica Sinica, 2023, 39(5): 2212027. |
| [2] | WAN J, WANG R, LIU Z, et al. A double-functional additive containing nucleophilic groups for high-performance Zn-ion batteries[J]. ACS Nano, 2023, 17(2): 1610-1621. |
| [3] | KOVENTHAN C, LO A Y. Morphology engineering of novel MnMoO4@NiMoO4 core-shell nanostructure as an electrode material for asymmetric supercapacitor device[J]. Chemical Engineering Journal, 2024, 485: 149950. |
| [4] | LI J, YANG Q-Q, HU Y-X, et al. Design of lamellar Mo2C nanosheets assembled by Mo2C nanoparticles as an anode material toward excellent sodium-ion capacitors[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(22): 18375-18383. |
| [5] | PAN H, WANG C, QIU M, et al. Mo doping and electrochemical activation Co‐induced vanadium composite as high‐rate and long‐life anode for Ca-ion batteries[J]. Energy & Environmental Materials, 2024, 7(5): e12690. |
| [6] | RUAN Q, QIAN Y, XUE M, et al. Emerging two-dimensional Mo-based materials for rechargeable metal-ion batteries: Advances and perspectives[J]. Journal of Energy Chemistry, 2024, 89: 487-518. |
| [7] | CHEN M, WANG Z, WANG Y, et al. Sodium-ion storage mechanisms and design strategies of molybdenum-based materials: A review[J]. Applied Materials Today, 2021, 23: 100985. |
| [8] | JIANG G, QIU Y, LU Q, et al. Mesoporous thin-wall molybdenum nitride for fast and stable Na/Li storage[J]. ACS Applied Materials & Interfaces, 2019, 11(44): 41188-41195. |
| [9] | JIANG G, XU F, YANG S, et al. Mesoporous, conductive molybdenum nitride as efficient sulfur hosts for high-performance lithium-sulfur batteries[J]. Journal of Power Sources, 2018, 395: 77-84. |
| [10] | YIN Y, FAN L, ZHANG Y, et al. MoP hollow nanospheres encapsulated in 3D reduced graphene oxide networks as high rate and ultralong cycle performance anodes for sodium-ion batteries[J]. Nanoscale, 2019, 11(15): 7129-7134. |
| [11] | CHEN A, ZHAO C, GUO Z, et al. Fast-growing multifunctional ZnMoO4 protection layer enable dendrite-free and hydrogen-suppressed Zn anode[J]. Energy Storage Materials, 2022, 44: 353-359. |
| [12] | XIE F, CHOY W C H, WANG C, et al. Low‐temperature solution‐processed hydrogen molybdenum and vanadium bronzes for an efficient hole‐transport layer in organic electronics[J]. Advanced Materials, 2013, 25(14): 2051-2055. |
| [13] | FREUND C, ABRANTES M, KüHN F E. Monomeric cyclopentadiene molybdenum oxides and their carbonyl precursors as epoxidation catalysts[J]. Journal of Organometallic Chemistry, 2006, 691(18): 3718-3729. |
| [14] | LI H, MCRAE L, FIRBY C J, et al. Rechargeable aqueous electrochromic batteries utilizing Ti-substituted tungsten molybdenum oxide based Zn2+ ion intercalation cathodes[J]. Advanced Materials, 2019, 31(15): 1807065. |
| [15] | JIANG Y, WANG Y, NI J, et al. Molybdenum‐based materials for sodium‐ion batteries[J]. InfoMat, 2021, 3(4): 339-352. |
| [16] | RESSLER T. Bulk structural investigation of the reduction of MoO3 with propene and the oxidation of MoO2 with oxygen[J]. Journal of Catalysis, 2002, 210(1): 67-83. |
| [17] | LI J, WANG C, JI C, et al. Molybdenum-based materials for alkali metal-ion batteries: Recent advances and perspectives[J]. Coordination Chemistry Reviews, 2024, 506: 215725. |
| [18] | WANG B, YAN J, ZHANG Y, et al. In situ carbon insertion in laminated molybdenum dioxide by interlayer engineering toward ultrastable "rocking-chair" zinc-ion batteries[J]. Advanced Functional Materials, 2021, 31(30): 2102827. |
| [19] | LIU R, FENG J, TANG R, et al. Long-range disordered MoO2 with rich oxygen vacancies for high-rate and durable lithium storage [J]. Chemical Engineering Journal, 2023, 468: 143766. |
| [20] | KIM W I, YEON J S, PARK H, et al. Polyoxometalate derived hierarchically structured N,P-Codoped reduced graphene oxide/MoO2 composites for high performance lithium-sulfur batteries [J]. Composites Part B: Engineering, 2023, 264: 110886. |
| [21] | FAN H, LI X, ZHOU J, et al. Realizing the long lifespan of molybdenum trioxide in aqueous aluminum ion batteries through potential regulation [J]. Renewables, 2023, 1(4): 455-464. |
| [22] | LEE S H, KIM Y H, DESHPANDE R, et al. Reversible lithium‐ion insertion in molybdenum oxide nanoparticles[J]. Advanced Materials, 2008, 20(19): 3627-3632. |
| [23] | SAHU S R, RIKKA V R, HARIDOSS P, et al. A novel α-MoO3/single‐walled carbon nanohorns composite as high‐performance anode material for fast‐charging lithium‐ion battery[J]. Advanced Energy Materials, 2020, 10(36): 2001627. |
| [24] | WANG B, ANG E H, YANG Y, et al. Interlayer engineering of molybdenum trioxide toward high‐capacity and stable sodium ion half/full batteries[J]. Advanced Functional Materials, 2020, 30(28): 2001708. |
| [25] | XU T, XU Z, YAO T, et al. Discovery of fast and stable proton storage in bulk hexagonal molybdenum oxide[J]. Nature Communications, 2023, 14(1): 8360. |
| [26] | LAKSHMY S, MONDAL B, KALARIKKAL N, et al. Recent developments in synthesis, properties, and applications of 2D Janus MoSSe and MoSe S(1-) alloys[J]. Advanced Powder Materials, 2024, 3(4): 100204. |
| [27] | DAI H, WANG L, ZHAO Y, et al. Recent advances in molybdenum-based materials for lithium-sulfur batteries[J]. Research, 2021, 2021: 5130420. |
| [28] | LIU Y, WANG H, CHENG L, et al. TiS2 nanoplates: A high-rate and stable electrode material for sodium ion batteries[J]. Nano Energy, 2016, 20: 168-175. |
| [29] | YANG W, WANG J, SI C, et al. Tungsten diselenide nanoplates as advanced lithium/sodium ion electrode materials with different storage mechanisms[J]. Nano Research, 2017, 10(8): 2584-2598. |
| [30] | ZHAI W, LI Z, WANG Y, et al. Phase engineering of nanomaterials: Transition metal dichalcogenides[J]. Chemical Reviews, 2024, 124(7): 4479-4539. |
| [31] | WANG W, BI H, LI C, et al. Edge-terminated few-layer MoS2 nanoflakes supported on TNAs@C with enhanced electrocatalysis activity for iodine reduction reaction[J]. Materials Today Nano, 2019, 6: 100033. |
| [32] | ZHOU Y, LIU Y, ZHANG M, et al. Rationally designed hierarchical N, P co-doped carbon connected 1T/2H-MoS2 heterostructures with cooperative effect as ultrafast and durable anode materials for efficient sodium storage[J]. Chemical Engineering Journal, 2022, 433: 133778. |
| [33] | WEI X, LIN C-C, WU C, et al. Three-dimensional hierarchically porous MoS2 foam as high-rate and stable lithium-ion battery anode[J]. Nature Communications, 2022, 13(1): 6006. |
| [34] | WANG Y, WANG K, ZHANG C, et al. Solvent‐exchange strategy toward aqueous dispersible MoS2 nanosheets and their nitrogen‐rich carbon sphere nanocomposites for efficient lithium/sodium ion storage[J]. Small, 2019, 15(45): 1903816. |
| [35] | LIM H, YU S, CHOI W, et al. Hierarchically designed nitrogen-doped MoS2/silicon oxycarbide nanoscale heterostructure as high-performance sodium-ion battery anode[J]. ACS Nano, 2021, 15(4): 7409-7420. |
| [36] | HE Y, LIU C, XIE Z, et al. Construction of cobalt sulfide/molybdenum disulfide heterostructure as the anode material for sodium ion batteries[J]. Advanced Composites and Hybrid Materials, 2023, 6(3): 85. |
| [37] | WU X, LIU H, GENG Y, et al. Interface-engineered molybdenum disulfide/porous graphene microfiber for high electrochemical energy storage[J]. Energy Storage Materials, 2023, 54: 30-39. |
| [38] | CHEN X, ZHANG A, ZOU H, et al. Defect engineering modulated MoSe2 cathode achieves highly effective photo-responsive zinc ion battery[J]. Energy Storage Materials, 2024, 70: 103457. |
| [39] | LI Y, WANG M, SUN J. Molecular engineering strategies toward molybdenum diselenide design for energy storage and conversion [J]. Advanced Energy Materials, 2022, 12(45): 2202600. |
| [40] | AI Y, WU S C, WANG K, et al. Three-dimensional molybdenum diselenide helical nanorod arrays for high-performance aluminum-ion batteries[J]. ACS Nano, 2020, 14(7): 8539-8550. |
| [41] | DU Y, ZHANG B, ZHOU W, et al. Laser-radiated tellurium vacancies enable high-performance telluride molybdenum anode for aqueous zinc-ion batteries[J]. Energy Storage Materials, 2022, 51: 29-37. |
| [42] | KIM E-K, YOON S J, BUI H T, et al. Epitaxial electrodeposition of single crystal MoTe2 nanorods and Li+ storage feasibility[J]. Journal of Electroanalytical Chemistry, 2020, 878: 114672. |
| [43] | PANDA M R, RAJ K A, GHOSH A, et al. Blocks of molybdenum ditelluride: A high rate anode for sodium-ion battery and full cell prototype study[J]. Nano Energy, 2019, 64: 103951. |
| [44] | PANDA M R, GANGWAR R, MUTHURAJ D, et al. High performance lithium-ion batteries using layered 2H‐MoTe2 as anode[J]. Small, 2020, 16(38): 2002669. |
| [45] | YANG Y, ZHONG Y, SHI Q, et al. Electrocatalysis in lithium sulfur batteries under lean electrolyte conditions[J]. Angewandte Chemie International Edition, 2018, 57(47): 15549-15552. |
| [46] | ZHOU X, LI L, YANG J, et al. Cobalt and molybdenum carbide nanoparticles grafted on nitrogen-doped carbon nanotubes as efficient chemical anchors and polysulfide conversion catalysts for lithium-sulfur batteries[J]. ChemElectroChem, 2020, 7(18): 3767-3775. |
| [47] | LI W, CHEN K, XU Q, et al. Mo2C/C hierarchical double-shelled hollow spheres as sulfur host for advanced Li-S batteries[J]. Angewandte Chemie International Edition, 2021, 60(39): 21512-21520. |
| [48] | HU T, HU Y, YAO T, et al. Freestanding molybdenum carbide nanowires electrode for high specific capacity and superior rate performance Li-CO2 batteries[J]. Energy Storage Materials, 2024, 72: 103740. |
| [49] | LIU F, HE J, LIU X, et al. MoC nanoclusters anchored Ni@N-doped carbon nanotubes coated on carbon fiber as three-dimensional and multifunctional electrodes for flexible supercapacitor and self‐heating device[J]. Carbon Energy, 2020, 3(1): 129-141. |
| [50] | JOSHI S, WANG Q, PUNTAMBEKAR A, et al. Facile synthesis of large area two-dimensional layers of transition-metal nitride and their use as insertion electrodes[J]. ACS Energy Letters, 2017, 2(6): 1257-1262. |
| [51] | JIANG Y, DONG J, TAN S, et al. Surface pseudocapacitance of mesoporous Mo3N2 nanowire anode toward reversible high-rate sodium-ion storage[J]. Journal of Energy Chemistry, 2021, 55: 295-303. |
| [52] | LIU Z, LIAN R, WU Z, et al. Ordered dual-channel carbon embedded with molybdenum nitride catalytically induced high-performance lithium-sulfur battery[J]. Chemical Engineering Journal, 2022, 431: 134163. |
| [53] | YI Z, LIU Y, LI Y, et al. Flexible membrane consisting of MoP ultrafine nanoparticles highly distributed inside N and P codoped carbon nanofibers as high‐performance anode for potassium‐ion batteries[J]. Small, 2019, 16(2): 1905301. |
| [54] | CHEN D, CHEN C, YU H, et al. Formation of N-doped carbon nanofibers decorated with MoP nanoflakes for dendrite-free lithium metal anode[J]. Advanced Functional Materials, 2024, 34(38): 2402951. |
| [55] | ZANG M, XU N, CAO G, et al. Cobalt molybdenum oxide derived high-performance electrocatalyst for the hydrogen evolution reaction[J]. ACS Catalysis, 2018, 8(6): 5062-5069. |
| [56] | XIA W, LUAN X, ZHANG W, et al. Sulfuration of hierarchical Mn-doped NiCo LDH heterostructures as efficient electrocatalyst for overall water splitting[J]. International Journal of Hydrogen Energy, 2023, 48(71): 27631-27641. |
| [57] | XU Z, SUN S, HAN Y, et al. High-energy-density asymmetric supercapacitor based on a durable and stable manganese molybdate nanostructure electrode for energy storage systems[J]. ACS Applied Energy Materials, 2020, 3(6): 5393-5404. |
| [58] | LIU Y, LI Y, LIU Z, et al. Uniform P-doped MnMoO4 nanosheets for enhanced asymmetric supercapacitors performance[J]. Molecules, 2024, 29(9): 1988. |
| [59] | JIANG J, ZHOU W, LI W, et al. Construction of electron-interactive CoMoO4@CoP core-shell structure on boron-doped graphene aerogel as strongly interface coupled hybrid electrodes for high flexible supercapacitor[J]. Chemical Engineering Journal, 2024, 496: 154123. |
| [60] | CHEN F, JI S, LIU Q, et al. Rational design of hierarchically core-shell structured Ni3S2@NiMoO4 nanowires for electrochemical energy storage[J]. Small, 2018, 14(27): 1800791. |
| [61] | ZHANG H, LU C, HOU H, et al. Tuning the electrochemical performance of NiCo2O4@NiMoO4 core-shell heterostructure by controlling the thickness of the NiMoO4 shell[J]. Chemical Engineering Journal, 2019, 370: 400-408. |
| [62] | WANG C, LI X, SONG H, et al. In‐plane heterostructured MoN/MoC nanosheets with enhanced interfacial charge transfer for superior pseudocapacitive storage[J]. Advanced Functional Materials, 2023, 34(12): 2311040. |
| [63] | SUN Y, ZHOU Y, ZHU Y, et al. In-situ synthesis of petal-like MoO2@MoN/NF heterojunction as both an advanced binder-free anode and an electrocatalyst for lithium ion batteries and water splitting[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(10): 9153-9163. |
| [64] | TIAN Y, YANG X, NAUTIYAL A, et al. One-step microwave synthesis of MoS2/MoO3@graphite nanocomposite as an excellent electrode material for supercapacitors[J]. Advanced Composites and Hybrid Materials, 2019, 2(1): 151-161. |
| [65] | SUN X, YANG J, CHEN Y, et al. Interconnected MoO2/MoS2@NC nanosheets as anodes with high-rate and long-life for lithium-ion and sodium-ion batteries[J]. Chemical Engineering Journal, 2024, 495: 153418. |
| [66] | YU L, TAO X, SUN D, et al. In situ phase transformation to form MoO3-MoS2 heterostructure with enhanced printable sodium ion storage[J]. Advanced Functional Materials, 2024, 34(29): 2311471. |
| [67] | KOU Z, WANG T, GU Q, et al. Rational design of holey 2D nonlayered transition metal carbide/nitride heterostructure nanosheets for highly efficient water oxidation[J]. Advanced Energy Materials, 2019, 9(16): 1803768. |
| [68] | HOU C, WANG J, DU W, et al. One-pot synthesized molybdenum dioxide-molybdenum carbide heterostructures coupled with 3D holey carbon nanosheets for highly efficient and ultrastable cycling lithium-ion storage[J]. Journal of Materials Chemistry A, 2019, 7(22): 13460-13472. |
| [69] | CHEN J, LUO Y, ZHANG W, et al. Tuning interface bridging between MoSe2 and three-dimensional carbon framework by incorporation of MoC intermediate to boost lithium storage capability[J]. Nano-Micro Letters, 2020, 12(1): 1-13. |
| [70] | VIKRAMAN D, HUSSAIN S, KARUPPASAMY K, et al. Engineering the novel MoSe2-Mo2C hybrid nanoarray electrodes for energy storage and water splitting applications[J]. Applied Catalysis B: Environmental, 2020, 264: 118531. |
| [71] | GAO Y, ZHANG S, XU L, et al. Fast charge transport motivated by tunable Mo2C/Mo2N high-quality heterointerface for superior pseudocapacitive storage[J]. Journal of Energy Chemistry, 2023, 83: 465-477. |
| [1] | Hao SUN, Zuoxia XING, Weining WU, Mingqi LI, Zhi ZHU, Gaohan WANG. Research on configuration strategies for wind-solar-battery-hydrogen hybrid power plants considering electricity market trading mechanisms [J]. Energy Storage Science and Technology, 2025, 14(7): 2801-2812. |
| [2] | Shuai YUAN, Yujie CUI, Donghao CHENG, Feng TAI, Jinzhong WU. Statistical analysis of fire and explosion accidents in electrochemical energy-storage stations from 2017 to 2024 throughout the world [J]. Energy Storage Science and Technology, 2025, 14(6): 2362-2376. |
| [3] | Ye CHEN, Jin LI, Houfu WU, Shaoyu ZHANG, Yuxi CHU, Ping ZHUO. Analysis of thermal runaway propagation and explosion risk of a large battery module for energy storage [J]. Energy Storage Science and Technology, 2024, 13(8): 2803-2812. |
| [4] | Zheng KOU, Zhenlong WANG. Effect analysis of energy storage equipment in the field of power Internet of Things [J]. Energy Storage Science and Technology, 2024, 13(8): 2785-2787. |
| [5] | Lijun XU, Lihong XU, Fangyuxuan SONG. System fault monitoring and diagnostic analysis of electrochemical energy storage power stations [J]. Energy Storage Science and Technology, 2024, 13(8): 2788-2790. |
| [6] | Chu ZHANG, Dongcai CHEN, Xiangping CHEN, Yongxiang CAI. Economic benefit analysis of optimal allocation of energy storage in multiple application scenarios [J]. Energy Storage Science and Technology, 2024, 13(6): 2078-2088. |
| [7] | Jianhang YANG, Wenting FENG, Junwei HAN, Xinru WEI, Chenyu MA, Changming MAO, Linjie ZHI, Debin KONG. Recent advances in rechargeable Li/Na-Cl2 batteries: From material construction to performance evaluation [J]. Energy Storage Science and Technology, 2024, 13(6): 1824-1834. |
| [8] | Kun ZENG, Xiaoyan ZHENG, Huiling GONG, Bo ZOU, Kai CHEN, Zhongna YAN. Research progress in liquid metal batteries based on lithium negative electrodes [J]. Energy Storage Science and Technology, 2024, 13(1): 299-310. |
| [9] | Su YAN, Fangfang ZHONG, Junwei LIU, Mei DING, Chuankun JIA. Key materials and advanced characterization of high-energy-density flow battery [J]. Energy Storage Science and Technology, 2024, 13(1): 143-156. |
| [10] | Yimei OUYANG, Mengmeng ZHAO, Guiming ZHONG, Zhangquan PENG. Nuclear magnetic resonance spectroscopy for probing interfaces in electrochemical energy storage systems [J]. Energy Storage Science and Technology, 2024, 13(1): 157-166. |
| [11] | Libo ZHANG, Gege WANG. Topic identification, evolution, and risk analysis of electrochemical energy storage battery technology [J]. Energy Storage Science and Technology, 2023, 12(8): 2680-2692. |
| [12] | Guangjin ZHAO, Bowen LI, Yuxia HU, Ruifeng DONG, Fangfang WANG. Overview of the echelon utilization technology and engineering application of retired power batteries [J]. Energy Storage Science and Technology, 2023, 12(7): 2319-2332. |
| [13] | Zhixiang CHENG, Wei CAO, Bo HU, Yunfang CHENG, Xin LI, Lihua JIANG, Kaiqiang JIN, Qingsong WANG. Thermal runaway and explosion propagation characteristics of large lithium iron phosphate battery for energy storage station [J]. Energy Storage Science and Technology, 2023, 12(3): 923-933. |
| [14] | Xuanliang ZHANG, Ting HE, Wenlong ZHU, Shen WANG, Jianhua ZENG, Quan XU, Yingchun NIU. A SOH estimation model for energy storage batteries based on multiple cycle features [J]. Energy Storage Science and Technology, 2023, 12(11): 3488-3498. |
| [15] | Yang LIU, Weijun TENG, Qingfa GU, Xin SUN, Yuliang TAN, Zhijin FANG, Jianlin LI. Scaled-up diversified electrochemical energy storage LCOE and its economic analysis [J]. Energy Storage Science and Technology, 2023, 12(1): 312-318. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
