Energy Storage Science and Technology ›› 2022, Vol. 11 ›› Issue (5): 1368-1382.doi: 10.19799/j.cnki.2095-4239.2021.0513
• Energy Storage Materials and Devices • Previous Articles Next Articles
Chaochao WEI1,2( ), Chuang YU1(
), Chuang YU1( ), Zhongkai WU1,2, Linfeng PENG1,3, Shijie CHENG1, Jia XIE1(
), Zhongkai WU1,2, Linfeng PENG1,3, Shijie CHENG1, Jia XIE1( )
)
Received:2021-10-08
Revised:2021-11-08
Online:2022-05-05
Published:2022-05-07
Contact:
Chuang YU, Jia XIE
E-mail:weichaochao@hust.edu.cn;cyu2020@hust.edu.cn;xiejia@hust.edu.cn
CLC Number:
Chaochao WEI, Chuang YU, Zhongkai WU, Linfeng PENG, Shijie CHENG, Jia XIE. Research progress of Li3PS4 solid electrolyte[J]. Energy Storage Science and Technology, 2022, 11(5): 1368-1382.
Table 2
Ionic conductivities of Li3PS4 sulfide electrolytes"
| Composition | Conductivity(RT)/(S/cm) | Ref. |
|---|---|---|
| β-Li3PS4 | 1.60×10-4 | [ |
| Li3.06P0.98Zn0.02S3.98O0.02 | 1.12×10-3 | [ |
| 75Li2S·(23)P2S5-2P2O5 | 2.53×10-4 | [ |
| 75Li2S·23P2S5-2P2Se5 | 6.00×10-4 | [ |
90Li3PS4-10LLZO 98Li3PS4-2Al2O3 98Li3PS4-2SiO2 86.9Li3PS4-13.1LiAlS2 2Li3PS4-LiI | 2.40×10-4 2.28×10-4 1.84×10-4 6.00×10-4 6.30×10-4 | [ [ [ [ [ |

Fig. 2
(a) Schematic illustration of the O-driven transition from 2D to 3D transport behaviour in β-Li3PS4 and the improvement of the interfacial stability against Li by O doping, (b) The variation of room-temperature ionic conductivities and the average crystallite size with the value of x in 75Li2S·(25-x)P2S5-xP2O5, (c) Raman spectra and b the corresponding XRD patterns and (d) of the dried 75Li2S·25P2S5 sample and heat-treated 75Li2S·(25-x)P2S5-xP2O5 (x= 0, 1, 2, 3, 5 mol%) samples, (e) Model for the oxide filler’s effect on the parent Li3PS4(LPS)electrolyte, ‘A’ represents the addition of no oxide filler, ‘B’ represents the space-charge effect, and ‘C’ shows the blocking effect of the oxide filler"
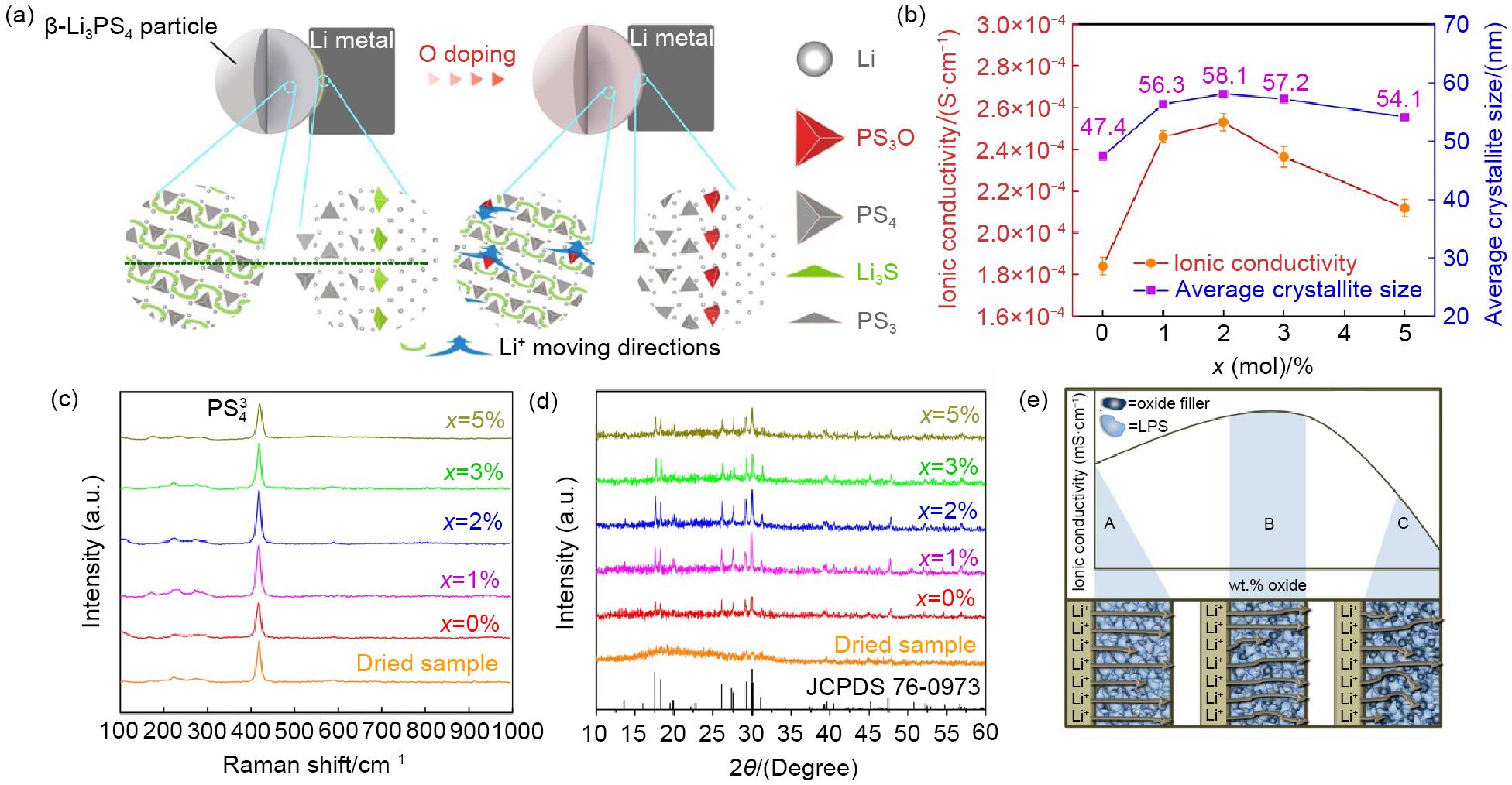
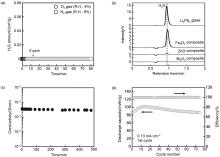
Fig. 3
(a) Time dependence of H2S amounts generated from the Li3PS4 glass under O2 or N2 gas flow, (b) H2S gas chro-matograms for the 90Li3PS4-10M x O y (M x O y : ZnO, Fe2O3,and Bi2O3) composites and the Li3PS4 glass, (c) Electrical conductivity of the pelletized 90Li3PS4-10ZnO composite as a function of exposure time to air, (d) Cycle performance of the In/LiCoO2 cell using the 90Li3PS4-10ZnO composite electrolyte[57]"

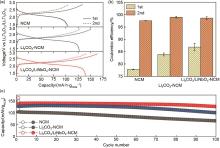
Fig. 4
(a) Initial and second cycle voltage profiles, (b) corresponding Coulombic efficiencies, and (c) cycling performance at a 0.1 C rate and 25 ℃ of SSB cells using bare (gray), Li2CO3-coated (blue), and Li2CO3/LiNbO3-coated NCM622 (red). Error bars in (b) indicate the standard deviation from two independent cells[66]"
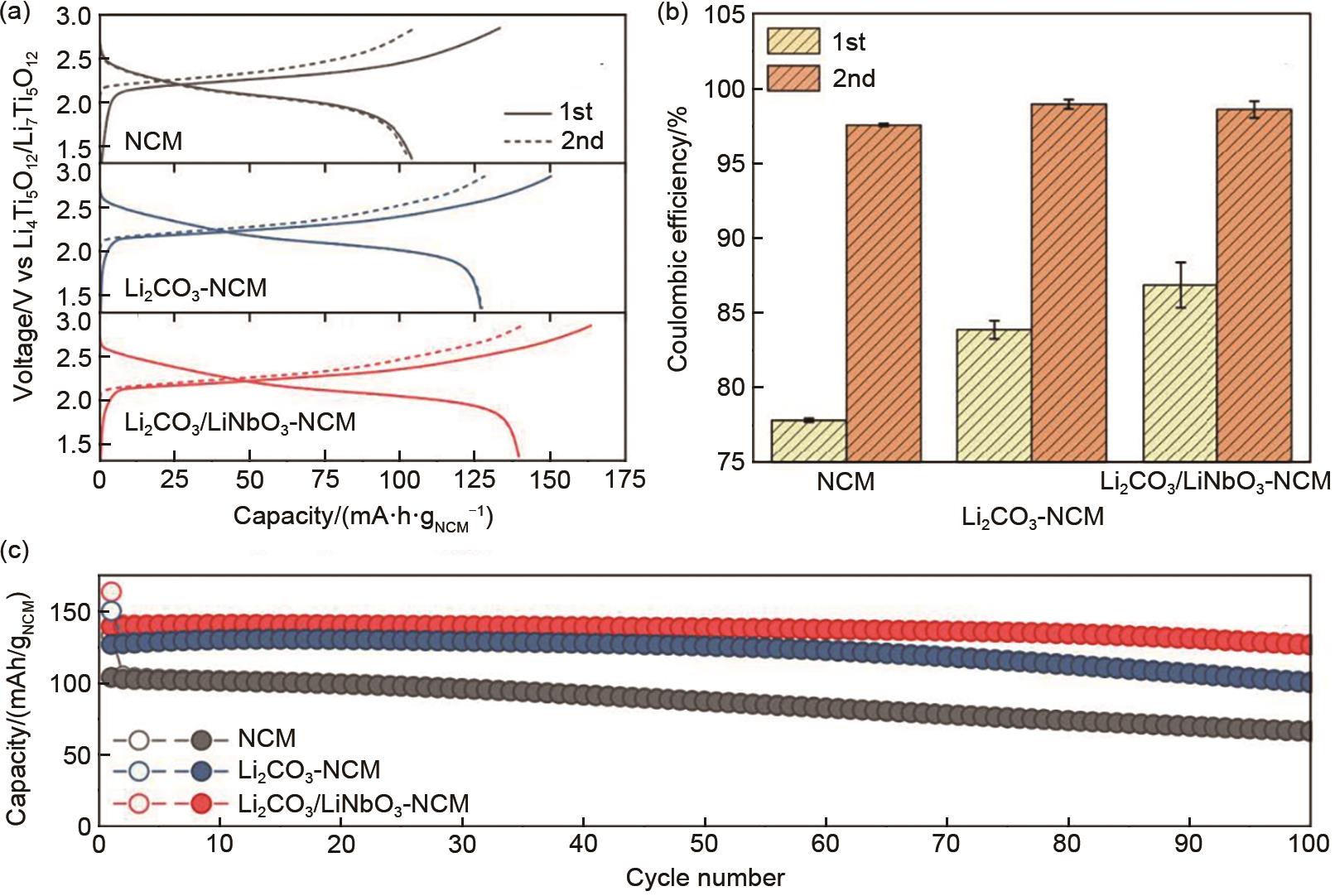
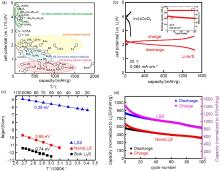
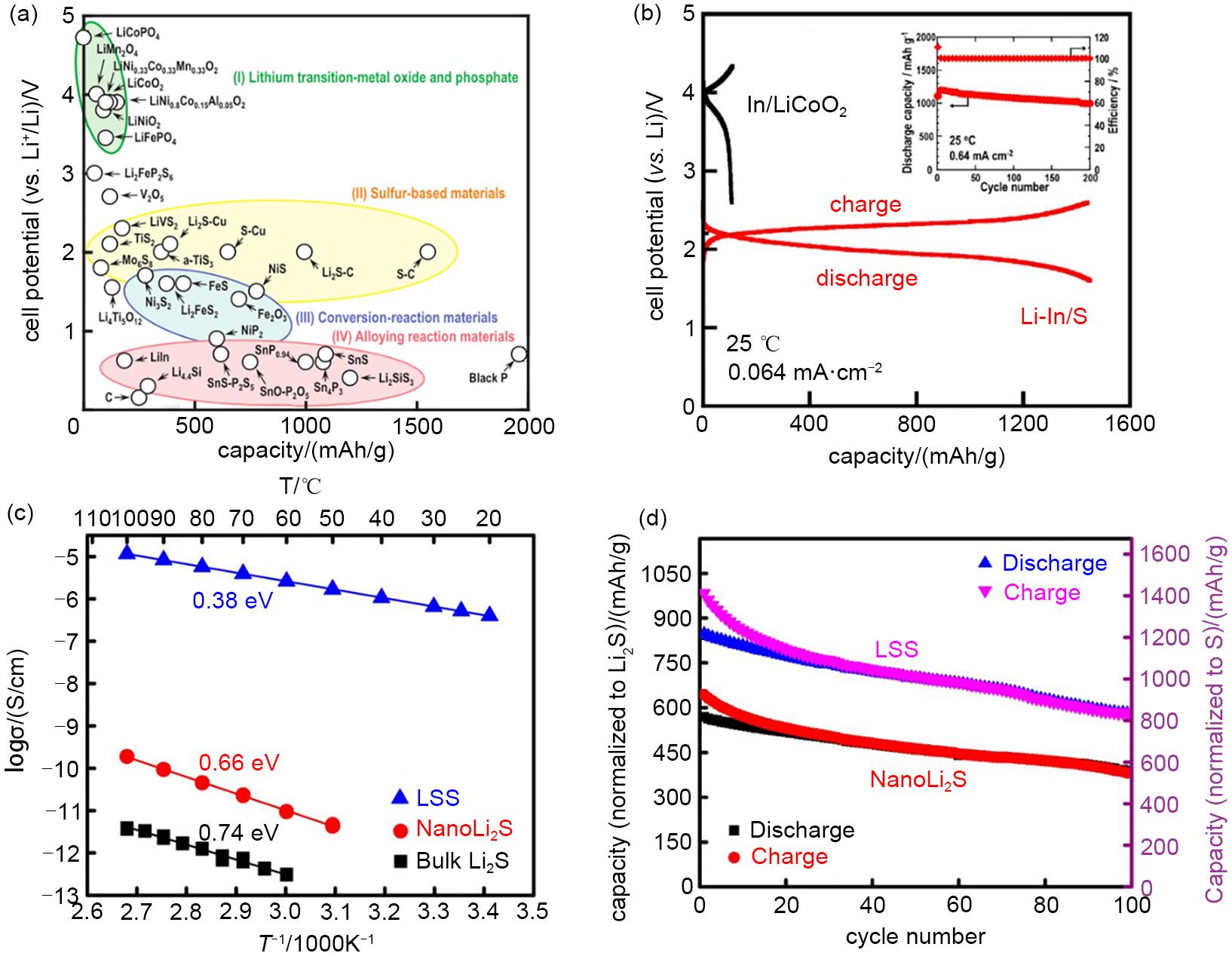
Fig. 5
(a) Correlation between cell potential (vs. Li+/Li) and reversible specific capacity in all-solid-state cells with a sulfide solid electrolyte reported so far, (b) Charge-discharge curves of an all-solid-state Li-In/S cell at 25 ℃ under the current density of 0.064 mA/cm2 (The embedded diagram shows the cycle performance of Li-In/S solid state battery at 0.64 mA/cm2), (c) Temperature dependency of ionic conductivities of the bulk Li2S, NanoLi2S, and LSS, (d) Cycling performance of LLS cell, NanoLi2S cell at 60 ℃ under the rate of C/10[77,80]"
| 1 | OH D Y, NAM Y J, PARK K H, et al. Slurry-fabricable Li+-conductive polymeric binders for practical all-solid-state lithium-ion batteries enabled by solvate ionic liquids[J]. Advanced Energy Materials, 2019, 9(16): 1802927. |
| 2 | TSUKASAKI H, MORI Y, OTOYAMA M, et al. Crystallization behavior of the Li2S-P2S5 glass electrolyte in the LiNi1/3Mn1/3Co1/3O2 positive electrode layer[J]. Scientific Reports, 2018, 8: 6214. |
| 3 | 许晓雄, 邱志军, 官亦标, 等. 全固态锂电池技术的研究现状与展望[J]. 储能科学与技术, 2013, 2(4): 331-341. |
| XU X X, QIU Z J, GUAN Y B, et al. All-solid-state lithium-ion batteries: State-of-the-art development and perspective[J]. Energy Storage Science and Technology, 2013, 2(4): 331-341. | |
| 4 | ITO Y, SAKUDA A, OHTOMO T, et al. Li4GeS4-Li3PS4 electrolyte thin films with highly ion-conductive crystals prepared by pulsed laser deposition[J]. Journal of the Ceramic Society of Japan, 2014, 122(1425): 341-345. |
| 5 | HOOD Z D, WANG H, LI Y C, et al. The "filler effect": A study of solid oxide fillers with β-Li3PS4 for lithium conducting electrolytes[J]. Solid State Ionics, 2015, 283: 75-80. |
| 6 | PENG L F, REN H T, ZHANG J Z, et al. LiNbO3-coated LiNi0.7Co0.1Mn0.2O2 and chlorine-rich argyrodite enabling high-performance solid-state batteries under different temperatures[J]. Energy Storage Materials, 2021, 43: 53-61. |
| 7 | TAKEUCHI T, KOJIMA T, KAGEYAMA H, et al. All-solid-state lithium-sulfur batteries using sulfurized alcohol composite material with improved coulomb efficiency[J]. Energy Technology, 2019, 7(12): 1900509. |
| 8 | 李泓, 许晓雄. 固态锂电池研发愿景和策略[J]. 储能科学与技术, 2016, 5(5): 607-614. |
| LI H, XU X X. R & D vision and strategies on solid lithium batteries[J]. Energy Storage Science and Technology, 2016, 5(5): 607-614. | |
| 9 | WEI X Y, DUWARD F S, et al. Highly conductive polymer electrolytes containing rigid polymers[J]. Chemistry of Materials, 10(9): 2307-2308. |
| 10 | REN H T, ZHANG Z Q, ZHANG J Z, et al. Improvement of stability and solid-state battery performances of annealed 70Li2S-30P2S5 electrolytes by additives[J]. Rare Metals, 2022, 41(1): 106-114. |
| 11 | LI J, CHEN H W, SHEN Y B, et al. Covalent interfacial coupling for hybrid solid-state Li ion conductor[J]. Energy Storage Materials, 2019, 23: 277-283. |
| 12 | LIU X Z, DING L, LIU Y Z, et al. Room-temperature ionic conductivity of Ba, Y, Al co-doped Li7La3Zr2O12 solid electrolyte after sintering[J]. Rare Metals, 2021, 40(8): 2301-2306. |
| 13 | PENG L F, YU C, ZHANG Z Q, et al. Chlorine-rich lithium argyrodite enabling solid-state batteries with capabilities of high voltage, high rate, low-temperature and ultralong cyclability[J]. Chemical Engineering Journal, 2022, 430: 132896. |
| 14 | 许阳阳, 李全国, 梁成都, 等. 硫化物固体电解质的研究进展[J]. 储能科学与技术, 2016, 5(5): 649-658. |
| XU Y Y, LI Q G, LIANG C D, et al. Research progress of solid electrolytes[J]. Energy Storage Science and Technology, 2016, 5(5): 649-658. | |
| 15 | JIN Y M, LIU C J, JIA Z G, et al. Building a highly functional Li1.3Al0.3Ti1.7(PO4)3/poly (vinylidene fluoride) composite electrolyte for all-solid-state lithium batteries[J]. Journal of Alloys and Compounds, 2021, 874: 159890. |
| 16 | LI Y B, SUN Y M, PEI A, et al. Robust pinhole-free Li3N solid electrolyte grown from molten lithium[J]. ACS Central Science, 2018, 4(1): 97-104. |
| 17 | YANG S P. An enhanced Li3AlH6 anode prepared by a solid-state ion exchange method for use in a solid-state lithium-ion battery[J]. International Journal of Electrochemical Science, 2020: 9487-9498. |
| 18 | ASANO T, SAKAI A, OUCHI S, et al. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries[J]. Advanced Materials, 2018, 30(44): 1803075. |
| 19 | ZHOU L, ASSOUD A, ZHANG Q, et al. New family of argyrodite thioantimonate lithium superionic conductors[J]. J Am Chem Soc, 2019, 141 (48): 19002-19013. |
| 20 | ZHANG Y B, CHEN R J, LIU T, et al. High capacity and superior cyclic performances of all-solid-state lithium batteries enabled by a glass-ceramics solo[J]. ACS Applied Materials & Interfaces, 2018, 10(12): 10029-10035. |
| 21 | ATARASHI A, TSUKASAKI H, OTOYAMA M, et al. Ex situ investigation of exothermal behavior and structural changes of the Li3PS4-LiNi1/3Mn1/3Co1/3O2 electrode composites[J]. Solid State Ionics, 2019, 342: 115046. |
| 22 | 吴敬华, 姚霞银. 基于硫化物固体电解质全固态锂电池界面特性研究进展[J]. 储能科学与技术, 2020, 9(2): 501-514. |
| WU J H, YAO X Y. Recent progress in interfaces of all-solid-state lithium batteries based on sulfide electrolytes[J]. Energy Storage Science and Technology, 2020, 9(2): 501-514. | |
| 23 | LU S T, KOSAKA F, SHIOTANI S, et al. Optimization of lithium ion conductivity of Li2S-P2S5 glass ceramics by microstructural control of crystallization kinetics[J]. Solid State Ionics, 2021, 362: 115583. |
| 24 | LIU Z Q, TANG Y F, WANG Y M, et al. High performance Li2S-P2S5 solid electrolyte induced by selenide[J]. Journal of Power Sources, 2014, 260: 264-267. |
| 25 | TOKUDA Y, UCHINO T, YOKO T. Ab initio study of NMR spectra of Li2S-SiS2 glass system[J]. Journal of Non-Crystalline Solids, 2003, 330(1/2/3): 61-65. |
| 26 | ZHAO R, HU G T, KMIEC S, et al. New amorphous oxy-sulfide solid electrolyte material: Anion exchange, electrochemical properties, and lithium dendrite suppression via in situ interfacial modification[J]. ACS Applied Materials & Interfaces, 2021, 13(23): 26841-26852. |
| 27 | ITOH K, SONOBE M, MORI K, et al. Structural observation of Li2S-GeS2 superionic glasses[J]. Physica B: Condensed Matter, 2006, 385/386: 520-522. |
| 28 | ITO Y, SAKUDA A, OHTOMO T, et al. Preparation of Li2S-GeS2 solid electrolyte thin films using pulsed laser deposition[J]. Solid State Ionics, 2013, 236: 1-4. |
| 29 | WANG C, LIANG J, ZHAO Y, et al. All-solid-state lithium batteries enabled by sulfide electrolytes: From fundamental research to practical engineering design[J]. Energ Environ Sci, 2021, 14 (5): 2577-2619. |
| 30 | WANG Z X, JIANG Y, WU J, et al. Reaction mechanism of Li2S-P2S5 system in acetonitrile based on wet chemical synthesis of Li7P3S11 solid electrolyte[J]. Chemical Engineering Journal, 2020, 393: 124706. |
| 31 | GARCIA-MENDEZ R, MIZUNO F, ZHANG R G, et al. Effect of processing conditions of 75Li2S-25P2S5 solid electrolyte on its DC electrochemical behavior[J]. Electrochimica Acta, 2017, 237: 144-151. |
| 32 | HOMMA K, YONEMURA M, NAGAO M, et al. Crystal structure of high-temperature phase of lithium ionic conductor, Li3PS4[J]. Journal of the Physical Society of Japan, 2010, 79(Suppl.A): 90-93. |
| 33 | HOMMA K, YONEMURA M, KOBAYASHI T, et al. Crystal structure and phase transitions of the lithium ionic conductor Li3PS4[J]. Solid State Ionics, 2011, 182(1): 53-58. |
| 34 | HAYASHI A, HAMA S, MINAMI T, et al. Formation of superionic crystals from mechanically milled Li2S-P2S5 glasses[J]. Electrochemistry Communications, 2003, 5(2): 111-114. |
| 35 | MERCIER R, MALUGANI J P, FAHYS B, et al. Superionic conduction in Li2S-P2S5-LiI-glasses[J]. Solid State Ionics, 1981, 5: 663-666. |
| 36 | KENNEDY J H. Ionically conductive glasses based on SiS2[J]. Materials Chemistry and Physics, 1989, 23(1/2): 29-50. |
| 37 | HAYASHI A, HAMA S, MORIMOTO H, et al. Preparation of Li2S-P2S5 amorphous solid electrolytes by mechanical milling[J]. Journal of the American Ceramic Society, 2001, 84(2): 477-479. |
| 38 | TACHEZ M, MALUGANI J P, MERCIER R, et al. Ionic conductivity of and phase transition in lithium thiophosphate Li3PS4[J]. Solid State Ionics, 1984, 14(3): 181-185. |
| 39 | KATO A, SUYAMA M, HOTEHAMA C, et al. High-temperature performance of all-solid-state lithium-metal batteries having Li/Li3PS4Interfaces modified with Au thin films[J]. Journal of the Electrochemical Society, 2018, 165(9): A1950-A1954. |
| 40 | TATSUMISAGO M, NAGAO M, HAYASHI A. Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable lithium batteries[J]. Journal of Asian Ceramic Societies, 2013, 1(1): 17-25. |
| 41 | TERAGAWA S, ASO K, TADANAGA K, et al. Preparation of Li2S-P2S5 solid electrolyte from N-methylformamide solution and application for all-solid-state lithium battery[J]. Journal of Power Sources, 2014, 248: 939-942. |
| 42 | TERAGAWA S, ASO K, TADANAGA K, et al. Liquid-phase synthesis of a Li3PS4 solid electrolyte using N-methylformamide for all-solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2014, 2(14): 5095. |
| 43 | GAMO H, NAGAI A, MATSUDA A, et al. The effect of solvent on reactivity of the Li2S-P2S5 system in liquid-phase synthesis of Li7P3S11 solid electrolyte[J]. Sci Rep-Uk, 2021, 11 (1):21097-21102. |
| 44 | PHUC N H H, TOTANI M, MORIKAWA K, et al. Preparation of Li3PS4 solid electrolyte using ethyl acetate as synthetic medium[J]. Solid State Ionics, 2016, 288: 240-243. |
| 45 | LIU Z C, FU W J, PAYZANT E A, et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4[J]. Journal of the American Chemical Society, 2013, 135(3): 975-978. |
| 46 | PHUC N H H, MUTO H, MATSUDA A. Fast preparation of Li3PS4 solid electrolyte using methyl propionate as synthesis medium[J]. Materials Today: Proceedings, 2019, 16: 216-219. |
| 47 | ARNOLD W, BUCHBERGER D A, LI Y, et al. Halide doping effect on solvent-synthesized lithium argyrodites Li6PS5X (X= Cl, Br, I) superionic conductors[J]. Journal of Power Sources, 2020, 464: 228158. |
| 48 | YUBUCHI S, UEMATSU M, HOTEHAMA C, et al. An argyrodite sulfide-based superionic conductor synthesized by a liquid-phase technique with tetrahydrofuran and ethanol[J]. Journal of Materials Chemistry A, 2019, 7(2): 558-566. |
| 49 | CHOI Y E, PARK K H, KIM D H, et al. Coatable Li4SnS4 solid electrolytes prepared from aqueous solutions for all-solid-state lithium-ion batteries[J]. ChemSusChem, 2017, 10(12): 2605-2611. |
| 50 | ITO A, KIMURA T, SAKUDA A, et al. Liquid-phase synthesis of Li3PS4 solid electrolyte using ethylenediamine[J]. Journal of Sol-Gel Science and Technology, 2022, 101(1): 2-7. |
| 51 | YAMAMOTO K, TAKAHASHI M, OHARA K, et al. Synthesis of sulfide solid electrolytes through the liquid phase: Optimization of the preparation conditions[J]. ACS Omega, 2020, 5(40): 26287-26294. |
| 52 | SUTO K, BONNICK P, NAGAI E, et al. Microwave-aided synthesis of lithium thiophosphate solid electrolyte[J]. Journal of Materials Chemistry A, 2018, 6(43): 21261-21265. |
| 53 | WANG X L, XIAO R J, LI H, et al. Oxygen-driven transition from two-dimensional to three-dimensional transport behaviour in β-Li3PS4 electrolyte[J]. Physical Chemistry Chemical Physics: PCCP, 2016, 18(31): 21269-21277. |
| 54 | LIU G Z, XIE D J, WANG X L, et al. High air-stability and superior lithium ion conduction of Li3+3 xP1- xZnxS4- xOx by aliovalent substitution of ZnO for all-solid-state lithium batteries[J]. Energy Storage Materials, 2019, 17: 266-274. |
| 55 | KIM J, YOON Y, EOM M, et al. Characterization of amorphous and crystalline Li2S-P2S5-P2Se5 solid electrolytes for all-solid-state lithium ion batteries[J]. Solid State Ionics, 2012, 225: 626-630. |
| 56 | LI J Y, LIU W M, ZHANG X F, et al. Heat treatment effects in oxygen-doped β-Li3PS4 solid electrolyte prepared by wet chemistry method[J]. Journal of Solid State Electrochemistry, 2021, 25(4): 1259-1269. |
| 57 | HOOD Z D, WANG H, LI Y C, et al. The "filler effect": A study of solid oxide fillers with β-Li3PS4 for lithium conducting electrolytes[J]. Solid State Ionics, 2015, 283: 75-80. |
| 58 | OOURA Y, MACHIDA N, NAITO M, et al. Electrochemical properties of the amorphous solid electrolytes in the system Li2S-Al2S3-P2S5[J]. Solid State Ionics, 2012, 225: 350-353. |
| 59 | RANGASAMY E, LIU Z C, GOBET M, et al. An iodide-based Li7P2S8I superionic conductor[J]. Journal of the American Chemical Society, 2015, 137(4): 1384-1387. |
| 60 | KANNO R, MURAYAMA M. Lithium ionic conductor thio-LISICON: The Li2S-GeS2-P2S5 system[J]. Journal of the Electrochemical Society, 2001, 148(7): 742-746. |
| 61 | LIU G Z, XIE D J, WANG X L, et al. High air-stability and superior lithium ion conduction of Li3+3 xP1- xZnxS4- xOx by aliovalent substitution of ZnO for all-solid-state lithium batteries[J]. Energy Storage Materials, 2019, 17: 266-274. |
| 62 | XU R C, XIA X H, WANG X L, et al. Tailored Li2S-P2S5glass-ceramic electrolyte by MoS2doping, possessing high ionic conductivity for all-solid-state lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2017, 5(6): 2829-2834. |
| 63 | XIE D J, CHEN S J, ZHANG Z H, et al. High ion conductive Sb2O5-doped β-Li3PS4 with excellent stability against Li for all-solid-state lithium batteries[J]. Journal of Power Sources, 2018, 389: 140-147. |
| 64 | CALPA M, ROSERO-NAVARRO N C, MIURA A, et al. Chemical stability of Li4PS4I solid electrolyte against hydrolysis[J]. Applied Materials Today, 2021, 22: 100918. |
| 65 | OHTOMO T, HAYASHI A, TATSUMISAGO M, et al. Characteristics of the Li2O-Li2S-P2S5 glasses synthesized by the two-step mechanical milling[J]. Journal of Non-Crystalline Solids, 2013, 364: 57-61. |
| 66 | HAYASHI A, MURAMATSU H, OHTOMO T, et al. Improvement of chemical stability of Li3PS4 glass electrolytes by adding MxOy (M=Fe, Zn, and Bi) nanoparticles[J]. Journal of Materials Chemistry A, 2013, 1(21): 6320. |
| 67 | KIMURA T, KATO A, HOTEHAMA C, et al. Preparation and characterization of lithium ion conductive Li3SbS4 glass and glass-ceramic electrolytes[J]. Solid State Ionics, 2019, 333: 45-49. |
| 68 | TAO Y C, CHEN S J, LIU D, et al. Lithium superionic conducting oxysulfide solid electrolyte with excellent stability against lithium metal for all-solid-state cells[J]. Journal of the Electrochemical Society, 2015, 163(2): A96-A101. |
| 69 | FAN X L, JI X, HAN F D, et al. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery[J]. Science Advances, 2018, 4(12): eaau9245. |
| 70 | MATSUDA R, HIRAHARA E, PHUC N H H, et al. Preparation of LiNi1/3Mn1/3Co1/3O2/Li3PS4 cathode composite particles using a new liquid-phase process and application to all-solid-state lithium batteries[J]. Journal of the Ceramic Society of Japan, 2018, 126(10): 826-831. |
| 71 | HARUYAMA J, SODEYAMA K, HAN L Y, et al. Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery[J]. Chemistry of Materials, 2014, 26(14): 4248-4255. |
| 72 | OHTA N, TAKADA K, ZHANG L, et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification[J]. Advanced Materials, 2006, 18(17): 2226-2229. |
| 73 | OHTA N, TAKADA K, SAKAGUCHI I, et al. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries[J]. Electrochemistry Communications, 2007, 9(7): 1486-1490. |
| 74 | SAKUDA A, KITAURA H, HAYASHI A, et al. Improvement of high-rate performance of all-solid-state lithium secondary batteries using LiCoO2 coated with Li2O-SiO2 glasses[J]. Electrochemical and Solid-State Letters, 2008, 11(1): A1. |
| 75 | KIM A Y, STRAUSS F, BARTSCH T, et al. Stabilizing effect of a hybrid surface coating on a Ni-rich NCM cathode material in all-solid-state batteries[J]. Chemistry of Materials, 2019, 31(23): 9664-9672. |
| 76 | CHEN S J, XIE D J, LIU G Z, et al. Sulfide solid electrolytes for all-solid-state lithium batteries: Structure, conductivity, stability and application[J]. Energy Storage Materials, 2018, 14: 58-74. |
| 77 | HAYASHI A, SAKUDA A, TATSUMISAGO M. Development of sulfide solid electrolytes and interface formation processes for bulk-type all-solid-state Li and Na batteries[J]. Frontiers in Energy Research, 2016, 4: 25. |
| 78 | ZHANG B H, LIU Y L, LIU J, et al. "Polymer-in-ceramic" based poly(Ɛ-caprolactone)/ceramic composite electrolyte for all-solid-state batteries[J]. Journal of Energy Chemistry, 2021, 52: 318-325. |
| 79 | ZHANG S N, ZENG Z, ZHAI W, et al. Bifunctional in situ polymerized interface for stable LAGP-based lithium metal batteries[J]. Advanced Materials Interfaces, 2021, 8(10): 2100072. |
| 80 | CHEN S J, WANG J Y, ZHANG Z H, et al. In-situ preparation of poly(ethylene oxide)/Li3PS4 hybrid polymer electrolyte with good nanofiller distribution for rechargeable solid-state lithium batteries[J]. Journal of Power Sources, 2018, 387: 72-80. |
| 81 | KATO A, YAMAMOTO M, SAKUDA A, et al. Mechanical properties of Li2S-P2S5 glasses with lithium halides and application in all-solid-state batteries[J]. ACS Applied Energy Materials, 2018, 1(3): 1002-1007. |
| 82 | YUE J, HUANG Y L, LIU S F, et al. Rational designed mixed-conductive sulfur cathodes for all-solid-state lithium batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(32): 36066-36071. |
| 83 | XIAO Y, YAMAMOTO K, MATSUI Y, et al. Comparison of sulfur cathode reactions between a concentrated liquid electrolyte system and a solid-state electrolyte system by soft X-ray absorption spectroscopy[J]. ACS Applied Energy Materials, 2021, 4(1): 186-193. |
| 84 | WU D S, ZHOU G M, MAO E Y, et al. A novel battery scheme: Coupling nanostructured phosphorus anodes with lithium sulfide cathodes[J]. Nano Research, 2020, 13(5): 1383-1388. |
| 85 | YANG H L, ZHANG B W, WANG Y X, et al. Alkali-metal sulfide as cathodes toward safe and high-capacity metal (M=Li, Na, K) sulfur batteries[J]. Advanced Energy Materials, 2020, 10(37): 2001764. |
| 86 | 苗力孝, 王维坤, 王梦佳, 等. 含单质硫正极复合材料[J]. 化学进展, 2013, 25(11): 1867-1875. |
| MIAO L X, WANG W K, WANG M J, et al. Sulfur composite cathode for lithium-sulfur batteries[J]. Progress in Chemistry, 2013, 25(11): 1867-1875. | |
| 87 | JIN C B, ZHANG W K, ZHUANG Z Z, et al. Enhanced sulfide chemisorption using boron and oxygen dually doped multi-walled carbon nanotubes for advanced lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2017, 5(2): 632-640. |
| 88 | KINOSHITA S, OKUDA K, MACHIDA N, et al. All-solid-state lithium battery with sulfur/carbon composites as positive electrode materials[J]. Solid State Ionics, 2014, 256: 97-102. |
| 89 | LIN Z, LIU Z C, DUDNEY N J, et al. Lithium superionic sulfide cathode for all-solid lithium-sulfur batteries[J]. ACS Nano, 2013, 7(3): 2829-2833. |
| 90 | MATSUYAMA T, HAYASHI A, HART C J, et al. Amorphous TiS3/S/C composite positive electrodes with high capacity for rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 2016, 163(8): A1730-A1735. |
| 91 | YE H L, MA L, ZHOU Y, et al. Amorphous MoS3 as the sulfur-equivalent cathode material for room-temperature Li-S and Na-S batteries[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(50): 13091-13096. |
| 92 | CUI T Y, XIAO Z H, WANG Z B, et al. FeS2/carbon felt as an efficient electro-Fenton cathode for carbamazepine degradation and detoxification: In-depth discussion of reaction contribution and empirical kinetic model[J]. Environmental Pollution, 2021, 282: 117023. |
| 93 | PAN M Y, HAKARI T, SAKUDA A, et al. Electrochemical properties of all-solid-state lithium batteries with amorphous FeSx-based composite positive electrodes prepared via mechanochemistry[J]. Electrochemistry, 2018, 86(4): 175-178. |
| 94 | FENG L W, LIU Y, WU L, et al. Surface modification with oxygen vacancy in LiNi0.5Co0.2Mn0.3O2 for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2021, 881: 160626. |
| 95 | XIE Z C, ZHANG Y Y, MIN X Q, et al. One-step bulk and surface co-modification of LiNi0.8Co0.15Al0.05O2 cathode material towards excellent long-term cyclability[J]. Electrochimica Acta, 2021, 379: 138124. |
| 96 | MACHIDA N, KASHIWAGI J, NAITO M, et al. Electrochemical properties of all-solid-state batteries with ZrO2-coated LiNi1/3Mn1/3Co1/3O2 as cathode materials[J]. Solid State Ionics, 2012, 225: 354-358. |
| 97 | KIM A Y, STRAUSS F, BARTSCH T, et al. Stabilizing effect of a hybrid surface coating on a Ni-rich NCM cathode material in all-solid-state batteries[J]. Chemistry of Materials, 2019, 31(23): 9664-9672. |
| 98 | STRAUSS F, BARTSCH T, DE BIASI L, et al. Impact of cathode material particle size on the capacity of bulk-type all-solid-state batteries[J]. ACS Energy Letters, 2018, 3(4): 992-996. |
| 99 | HAN L J, WEI Q H, CHEN H M, et al. Open-framework germanates derived GeO2/C nanocomposite as a long-life and high-capacity anode for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2021, 881: 160533. |
| 100 | ZHANG Y C, CHEN M Y, CHEN Z Y, et al. Constructing cycle-stable Si/TiSi2 composites as anode materials for lithium ion batteries through direct utilization of low-purity Si and Ti-bearing blast furnace slag[J]. Journal of Alloys and Compounds, 2021, 876: 160125. |
| 101 | OKUNO R, YAMAMOTO M, TERAUCHI Y, et al. Stable cyclability of porous Si anode applied for sulfide-based all-solid-state batteries[J]. Energy Procedia, 2019, 156: 183-186. |
| [1] | LI Yitao, SHEN Kaier, PANG Quanquan. Advance in organics enhanced sulfide-based solid-state batteries [J]. Energy Storage Science and Technology, 2022, 11(6): 1902-1918. |
| [2] | Liangtao XIONG, Jifen WANG, Huaqing XIE, Xuelai ZHANG. Effect of vacancy defects on thermal conductivity of single-layer graphene by molecular dynamics [J]. Energy Storage Science and Technology, 2022, 11(5): 1322-1330. |
| [3] | Suting WENG, Zepeng LIU, Gaojing YANG, Simeng ZHANG, Xiao ZHANG, Qiu FANG, Yejing LI, Zhaoxiang WANG, Xuefeng WANG, Liquan CHEN. Cryogenic electron microscopy (cryo-EM) characterizing beam-sensitive materials in lithium metal batteries [J]. Energy Storage Science and Technology, 2022, 11(3): 760-780. |
| [4] | Shiwei DENG, Jianfang WU, Tuo SHI. Defect chemistry analysis of solid electrolytes: Point defects in grain bulk and grain boundary space-charge layer [J]. Energy Storage Science and Technology, 2022, 11(3): 939-947. |
| [5] | Yongli TONG, Xiang WU. Electrochemical performance of Co3O4 electrode materials derived from Co metal-organic framework [J]. Energy Storage Science and Technology, 2022, 11(3): 1035-1043. |
| [6] | Yue SU, Xuhua LIU, Fanglei ZENG, Yurong REN, Bencai LIN. Preparation and properties of polyvinylidene fluoride/polyvinylidene fluoride sulfonate lithium/lithium salt composite solid electrolyte [J]. Energy Storage Science and Technology, 2021, 10(6): 2069-2076. |
| [7] | Zhuo XU, Lili ZHENG, Bing CHEN, Tao ZHANG, Xiuling CHANG, Shouli WEI, Zuoqiang DAI. Overview of research on composite electrolytes for solid-state batteries [J]. Energy Storage Science and Technology, 2021, 10(6): 2117-2126. |
| [8] | Bohui LU, Zhicheng SHI, Yongxue ZHANG, Hongyu ZHAO, Zixi WANG. Investigation of the charging and discharging performance of paraffin/nano-Fe3O4 composite phase change material in a shell and tube thermal energy storage unit [J]. Energy Storage Science and Technology, 2021, 10(5): 1709-1719. |
| [9] | Yanfang ZHAI, Guanming YANG, Wangshu HOU, Jianyao YAO, Zhaoyin WEN, Shufeng SONG, Ning HU. Solvothermal synthesis of three-dimensional petaloid garnet electrolyte and its application in solid polymer electrolytes [J]. Energy Storage Science and Technology, 2021, 10(3): 905-913. |
| [10] | Dangling LIU, Shimin WANG, Zhihui GAO, Lufu XU, Shubiao XIA, Hong GUO. Properties of three-dimensional NZSPO/PAN-[PEO-NATFST] sodium-battery-composite solid electrolyte [J]. Energy Storage Science and Technology, 2021, 10(3): 931-937. |
| [11] | Saisai ZHANG, Hailei ZHAO. Electrode/electrolyte interfaces in Li7La3Zr2O12 garnet-based solid-state lithium metal battery: Challenges and progress [J]. Energy Storage Science and Technology, 2021, 10(3): 863-871. |
| [12] | Yanming CUI, Zhihua ZHANG, Yuanqiao HUANG, Jiu LIN, Xiayin YAO, Xiaoxiong XU. Prototype all-solid-state battery electrodes preparation and assembly technology [J]. Energy Storage Science and Technology, 2021, 10(3): 836-847. |
| [13] | Xinxin ZHU, Wei JIANG, Zhengwei WAN, Shu ZHAO, Zeheng LI, Liguang WANG, Wenbin NI, Min LING, Chengdu LIANG. Research progress in electrolyte and interfacial issues of solid lithium sulfur batteries [J]. Energy Storage Science and Technology, 2021, 10(3): 848-862. |
| [14] | Peng ZHANG, Xingqiang LAI, Junrong SHEN, Donghai ZHANG, Yongheng YAN, Rui ZHANG, Jun SHENG, Kangwei DAI. Research and industrialization progress of solid-state lithium battery [J]. Energy Storage Science and Technology, 2021, 10(3): 896-904. |
| [15] | Chunyan YANG, Yunlong MA, Xiaoqiong FENG, Shiying ZHANG, Changsheng AN, Jingfeng LI. Research progress of carbon-based materials in aluminum-ion batteries [J]. Energy Storage Science and Technology, 2021, 10(2): 432-439. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
