Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (11): 4184-4198.doi: 10.19799/j.cnki.2095-4239.2025.0496
• Energy Storage Materials and Devices • Previous Articles Next Articles
Yuxuan JIN1( ), Quan ZHOU1(
), Quan ZHOU1( ), Lin ZHOU2(
), Lin ZHOU2( ), Teng GAO1, Zijie LI1, Yan WANG2, Junlong LU1
), Teng GAO1, Zijie LI1, Yan WANG2, Junlong LU1
Received:2025-05-27
Revised:2025-06-16
Online:2025-11-28
Published:2025-11-24
Contact:
Quan ZHOU, Lin ZHOU
E-mail:george_jyx@163.com;zhouquan@ioply.cn;lzhou@iphy.ac.cn
CLC Number:
Yuxuan JIN, Quan ZHOU, Lin ZHOU, Teng GAO, Zijie LI, Yan WANG, Junlong LU. Research progress of NASICON-type phosphate cathode materials for sodium-ion batteries[J]. Energy Storage Science and Technology, 2025, 14(11): 4184-4198.

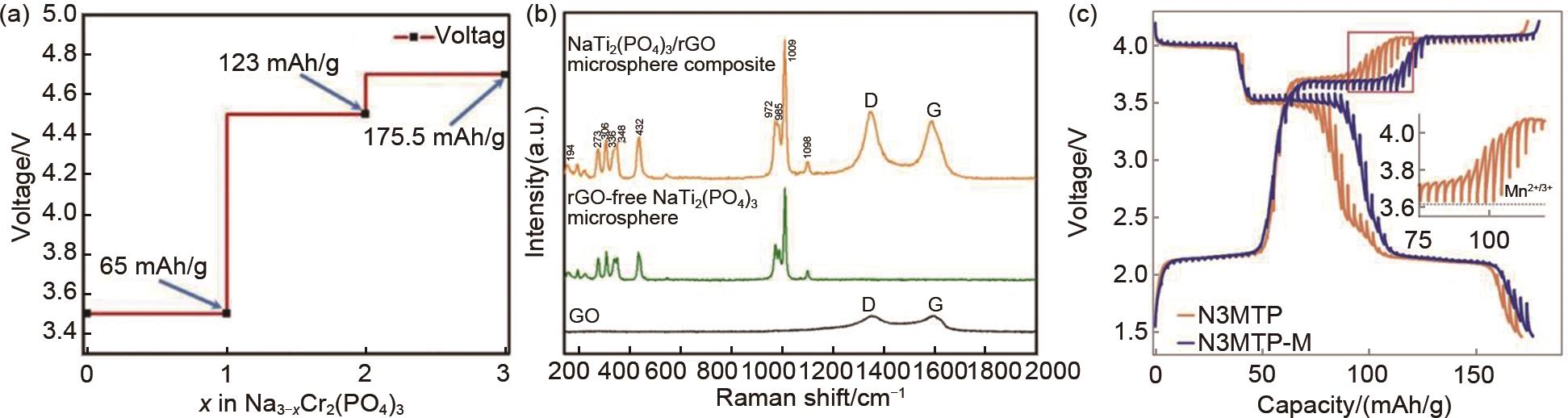
Fig. 4
(a) Influence of the number of deintercalated electrons on the voltage and specific capacity of Na3Cr2(PO4)3 material[33], (b) Raman spectrum of the synthesized NaTi2(PO4)3/rGO material[35], (c) Capacity fading of Na3MnTi(PO4)3 material caused by the Jahn-Teller effect during cycling[36]"
| [1] | PAN H L, HU Y S, CHEN L Q. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage[J]. Energy & Environmental Science, 2013, 6(8): 2338-2360. DOI: 10.1039/C3EE40847G. |
| [2] | QIAN J F, WU C, CAO Y L, et al. Prussian blue cathode materials for sodium-ion batteries and other ion batteries[J]. Advanced Energy Materials, 2018, 8(17): 1702619. DOI: 10.1002/aenm. 201702619. |
| [3] | XU L G, FENG X Y, JIA W B, et al. Recent advances and challenges of inverted lead-free tin-based perovskite solar cells[J]. Energy & Environmental Science, 2021, 14(8): 4292-4317. DOI: 10.1039/D1EE00890K. |
| [4] | YABUUCHI N, KOMABA S. Recent research progress on iron- and manganese-based positive electrode materials for rechargeable sodium batteries[J]. Science and Technology of Advanced Materials, 2014, 15(4): DOI: 10.1088/1468-6996/15/4/043501. |
| [5] | GOODENOUGH J B. NASICON I structure and conductivity[J]. Solid State Ionics, 1983, 9: 793-794. DOI: 10.1016/0167-2738(83)90088-7. |
| [6] | FEI W B, SUI Y L, WANG Y A, et al. Regulating Na/Mn antisite defects and reactivating anomalous jahn-teller behavior for Na4Fe1.5Mn1.5(PO4)2(P2O7) cathode material with superior performance[J]. ACS Nano, 2025, 19(8): 8303-8315. DOI: 10.1021/acsnano. 4c18614. |
| [7] | HAMEED A S, OHARA M, KUBOTA K, et al. A phosphite-based layered framework as a novel positive electrode material for Na-ion batteries[J]. Journal of Materials Chemistry A, 2021, 9(8): 5045-5052. DOI: 10.1039/D0TA10517A. |
| [8] | LIANG L W, LI X Y, ZHAO F, et al. High-rate cathodes: Construction and operating mechanism of high-rate Mo-doped Na3V2(PO4)3@C nanowires toward practicable wide-temperature-tolerance Na-ion and hybrid Li/Na-ion batteries[J]. Advanced Energy Materials, 2021, 11(21): 2170079. DOI: 10.1002/aenm.202170079. |
| [9] | LI F, ZHU Y E, SHENG J, et al. GO-induced preparation of flake-shaped Na3V2(PO4)3@rGO as high-rate and long-life cathodes for sodium-ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(48): 25276-25281. DOI: 10.1039/C7TA07943E. |
| [10] | PARK S, WANG Z L, CHOUDHARY K, et al. Obtaining V2(PO4)3 by sodium extraction from single-phase NaxV2(PO4)3 (1<x<3) positive electrode materials[J]. Nature Materials, 2024, 24(2): 234-242. DOI: 10.1038/s41563-024-02023-7. |
| [11] | SINGH B, WANG Z L, PARK S, et al. A chemical map of NaSICON electrode materials for sodium-ion batteries[J]. Journal of Materials Chemistry A, 2021, 9(1): 281-292. DOI: 10.1039/d0ta 10688g. |
| [12] | LI S H, ZHOU W, LIU F Y, et al. Mitigating long range Jahn-Teller ordering to stabilize Mn redox reaction in biphasic layered sodium oxide[J]. Advanced Energy Materials, 2025, 15(10): 2403955. DOI: 10.1002/aenm.202403955. |
| [13] | BEN YAHIA M, VERGNET J, SAUBANÈRE M, et al. Unified picture of anionic redox in Li/Na-ion batteries[J]. Nature Materials, 2019, 18(5): 496-502. DOI: 10.1038/s41563-019-0318-3. |
| [14] | PARK S, WANG Z L, DENG Z Y, et al. Crystal structure of Na2V2(PO4)3, an intriguing phase spotted in the Na3V2(PO4)3-Na1V2(PO4)3 system[J]. Chemistry of Materials, 2022, 34(1): 451-462. DOI: 10. 1021/acs.chemmater.1c04033. |
| [15] | JIAN Z L, HAN W Z, LU X, et al. Superior electrochemical performance and storage mechanism of Na3V2(PO4)3 cathode for room-temperature sodium-ion batteries[J]. Advanced Energy Materials, 2013, 3(2): 156-160. DOI: 10.1002/aenm.201200558. |
| [16] | SU Q, ZHOU Y F, ZHU J, et al. Nanoflake-assembled hierarchical Na3V2(PO4)3@C microspheres for ultrafast and highly durable sodium storage[J]. ACS Applied Energy Materials, 2023, 6(19): 10128-10136. DOI: 10.1021/acsaem.3c01871. |
| [17] | ZHU Q, WANG M, NAN B, et al. Core/shell nanostructured Na3V2(PO4)3/C/TiO2 composite nanofibers as a stable anode for sodium-ion batteries[J]. Journal of Power Sources, 2017, 362: 147-159. DOI: 10.1016/j.jpowsour.2017.07.004. |
| [18] | DONG L, LIU R X, FENG J M, et al. Improved high-rate performance of Na3V2(PO4)3 with an atomic layer deposition-generated Al2O3 layer as a cathode material for sodium-ion batteries[J]. Materials Letters, 2017, 205: 75-78. DOI: 10.1016/j.matlet.2017.06.067. |
| [19] | ZENG J, YANG Y, LAI S B, et al. A promising high-voltage cathode material based on mesoporous Na3V2(PO4)3/C for rechargeable magnesium batteries[J]. Chemistry-A European Journal, 2017, 23(66): 16898-16905. DOI: 10.1002/chem.201704303. |
| [20] | YAO X H, ZHU Z X, LI Q, et al. 3.0 V high energy density symmetric sodium-ion battery: Na4V2(PO4)3∥Na3V2(PO4)3[J]. ACS Applied Materials & Interfaces, 2018, 10(12): 10022-10028. DOI: 10.1021/acsami.7b16901. |
| [21] | XU M L, ZHANG F X, ZHANG Y H, et al. Controllable synthesis of a Na-enriched Na4V2(PO4)3 cathode for high-energy sodium-ion batteries: A redox-potential-matched chemical sodiation approach[J]. Chemical Science, 2023, 14(44): 12570-12581. DOI: 10.1039/D3SC 03498D. |
| [22] | YANG Y, XU G R, TANG A P, et al. Na3V2(PO4)3-decorated Na3V2(PO4)2F3 as a high-rate and cycle-stable cathode material for sodium ion batteries[J]. RSC Advances, 2024, 14(17): 11862-11871. DOI: 10.1039/D4RA01653J. |
| [23] | HU P, WANG X F, WANG T S, et al. Boron substituted Na3V2(P1- xBxO4)3 cathode materials with enhanced performance for sodium-ion batteries[J]. Advanced Science, 2016, 3(12): 1600112. DOI: 10.1002/advs.201600112. |
| [24] | WANG D, WU Y B, LV J M, et al. Carbon encapsulated maricite NaFePO4 nanoparticles as cathode material for sodium-ion batteries[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 583: 123957. DOI: 10.1016/j.colsurfa. 2019.123957. |
| [25] | CAO Y J, LIU Y, ZHAO D Q, et al. Highly stable Na3Fe2(PO4)3@Hard carbon sodium-ion full cell for low-cost energy storage[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(3): 1380-1387. DOI: 10.1021/acssuschemeng.9b05098. |
| [26] | KIM H, PARK I, LEE S, et al. Understanding the electrochemical mechanism of the new iron-based mixed-phosphate Na4Fe3(PO4)2(P2O7) in a Na rechargeable battery[J]. Chemistry of Materials, 2013, 25(18): 3614-3622. DOI: 10.1021/cm4013816. |
| [27] | SENTHILKUMAR B, RAMBABU A, MURUGESAN C, et al. Iron-based mixed phosphate Na4Fe3(PO4)2P2O7 thin films for sodium-ion microbatteries[J]. ACS Omega, 2020, 5(13): 7219-7224. DOI: 10.1021/acsomega.9b03835. |
| [28] | HUANG J R, ZHANG Z H, CHEN D Q, et al. Spray-drying synthesis of Na4Fe3(PO4)2P2O7@CNT cathode for ultra-stable and high-rate sodium-ion batteries[J]. Molecules, 2025, 30(3): 753. DOI: 10.3390/molecules30030753. |
| [29] | LI Z, ZHANG Y, ZHANG J H, et al. Sodium-ion battery with a wide operation-temperature range from -70 to 100 ℃[J]. Angewandte Chemie International Edition, 2022, 61(13): e202116930. DOI: 10. 1002/anie.202116930. |
| [30] | ZHAO A L, YUAN T C, LI P, et al. A novel Fe-defect induced pure-phase Na4Fe2.91(PO4)2P2O7 cathode material with high capacity and ultra-long lifetime for low-cost sodium-ion batteries[J]. Nano Energy, 2022, 91: 106680. DOI: 10.1016/j.nanoen.2021.106680. |
| [31] | ZHANG J H, TANG L B, ZHANG Y, et al. Polyvinylpyrrolidone assisted synthesized ultra-small Na4Fe3(PO4)2(P2O7) particles embedded in 1D carbon nanoribbons with enhanced room and low temperature sodium storage performance[J]. Journal of Power Sources, 2021, 498: 229907. DOI: 10.1016/j.jpowsour. 2021.229907. |
| [32] | SUBAŞı Y, ALTENSCHMIDT L, LINDGREN F, et al. Synthesis and characterization of a crystalline Na4Fe3(PO4)2(P2O7) cathode material for sodium-ion batteries[J]. Journal of Materials Chemistry A, 2024, 12(35): 23506-23517. DOI: 10.1039/D4TA 03554B. |
| [33] | MAMOOR M, LIAN R Q, WANG D S, et al. Identification of the structural, electronic properties, and ionic diffusion kinetics of Na3Cr2(PO4)3 by first-principles calculations[J]. Electrochimica Acta, 2021, 379: 138157. DOI: 10.1016/j.electacta.2021.138157. |
| [34] | KAWAI K, ASAKURA D, NISHIMURA S I, et al. Stabilization of a 4.5 V Cr4+/Cr3+ redox reaction in NASICON-type Na3Cr2(PO4)3 by Ti substitution[J]. Chemical Communications, 2019, 55(91): 13717-13720. DOI: 10.1039/C9CC04860J. |
| [35] | ROH H K, KIM M S, CHUNG K Y, et al. A chemically bonded NaTi2(PO4)3/rGO microsphere composite as a high-rate insertion anode for sodium-ion capacitors[J]. Journal of Materials Chemistry A, 2017, 5(33): 17506-17516. DOI: 10.1039/C7TA05252A. |
| [36] | WANG Y A, SUI Y L, XIAO Z, et al. Inner/interface engineered iron/manganese-based mixed phosphate cathode with high energy density and ultra-long cycle life for sodium-ion batteries[J]. Advanced Functional Materials, 2025, 35(28): 2500290. DOI: 10.1002/adfm.202500290. |
| [37] | LIU Y, RONG X H, BAI R, et al. Identifying the intrinsic anti-site defect in manganese-rich NASICON-type cathodes[J]. Nature Energy, 2023, 8(10): 1088-1096. DOI: 10.1038/s41560-023-01301-z. |
| [38] | LALÈRE F, SEZNEC V, COURTY M, et al. Improving the energy density of Na3V2(PO4)3-based positive electrodes through V/Al substitution[J]. Journal of Materials Chemistry A, 2015, 3(31): 16198-16205. DOI: 10.1039/C5TA03528G. |
| [39] | XU C L, ZHAO J M, WANG Y A, et al. Reversible activation of V4+/V5+ redox couples in NASICON phosphate cathodes[J]. Advanced Energy Materials, 2022, 12(25): 2200966. DOI: 10.1002/aenm. 202200966. |
| [40] | MAI B, XING B Y, YUE Y F, et al. Cr-doped Na3V2(PO4)3@C enables high-capacity with V2+/V5+ reaction and stable sodium storage[J]. Journal of Materials Science & Technology, 2023, 165: 1-7. DOI: 10.1016/j.jmst.2023.05.005. |
| [41] | BAG S, MURARKA H, ZHOU C T, et al. Understanding the Na-ion storage mechanism in Na3+ xV2- xMx(PO4)3 (M = Ni2+, Co2+, Mg2+; x = 0.1-0.5) cathodes[J]. ACS Applied Energy Materials, 2020, 3(9): 8475-8486. DOI: 10.1021/acsaem.0c01118. |
| [42] | INOISHI A, YOSHIOKA Y, ZHAO L W, et al. Improvement in the energy density of Na3V2(PO4)3 by Mg substitution[J]. ChemElectroChem, 2017, 4(11): 2755-2759. DOI: 10.1002/celc. 201700540. |
| [43] | AHSAN M T, QIU D P, ALI Z, et al. Unraveling the fast Na diffusion kinetics of NASICON at high voltage via high entropy for sodium-ion battery[J]. Advanced Energy Materials, 2024, 14(4): 2302733. DOI: 10.1002/aenm.202302733. |
| [44] | LIN B H, WU X, HE X J, et al. High-entropy doping in NASICON cathodes: Activating the V4+/V5+ redox couple and inducing a reversible single solid-solution phase reaction for advanced sodium ion batteries[J]. Journal of Colloid and Interface Science, 2025, 690: 137299. DOI: 10.1016/j.jcis.2025.137299. |
| [45] | ZHANG H, GU Z Y, WANG X T, et al. Electronic confinement-restrained Mn·Na anti-site defects in sodium-rich phosphates toward multi-electron transfer and high energy efficiency[J]. Advanced Materials, 2024, 36(47): 2410797. DOI: 10.1002/adma.202410797. |
| [46] | WU X N, LI Z K, ZHANG J C, et al. Insight into highly reversible multielectron V3+/V4+/V5+ reaction of high-entropy doped NASICON cathode for sodium ion batteries[J]. ACS Applied Materials & Interfaces, 2025, 17(8): 12227-12236. DOI: 10.1021/acsami.4c21356. |
| [47] | XIE P X, ZHAO Q Y, DONG H J, et al. Reversible V4+/V5+ redox in Na4VFe(PO4)3 cathode for high-power sodium-ion batteries[J]. Chemical Engineering Journal, 2025, 509: 161209. DOI: 10.1016/j.cej.2025.161209. |
| [48] | ZHU L, WANG M M, LIANG K, et al. Achieving high capacity and ultra-stable sodium storage of Na2TiV(PO4)3 cathode by integrated lattice regulation and surface modification[J]. Journal of Energy Chemistry, 2025, 103: 793-802. DOI: 10.1016/j.jechem.2024. 10.052. |
| [49] | RUBIO S, LIU R, LIU X S, et al. Exploring the high-voltage Mg2+/Na+ co-intercalation reaction of Na3VCr(PO4)3 in Mg-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(30): 18081-18091. DOI: 10.1039/C9TA05608D. |
| [50] | PARK J S, KIM J, JO J H, et al. Role of the Mn substituent in Na3V2(PO4)3 for high-rate sodium storage[J]. Journal of Materials Chemistry A, 2018, 6(34): 16627-16637. DOI: 10.1039/C8TA 06162A. |
| [51] | ZHOU Y F, GUO S, LI S, et al. Enabling high-performance Na4MnV(PO4)3 cathode via synergetic strategy of carbon encapsulation and nanoengineering[J]. Journal of Power Sources, 2022, 521: 230974. DOI: 10.1016/j.jpowsour.2022. 230974. |
| [52] | PARK S, CHOTARD J N, CARLIER D, et al. Irreversible electrochemical reaction at high voltage induced by distortion of Mn and V structural environments in Na4MnV(PO4)3[J]. Chemistry of Materials, 2023, 35(8): 3181-3195. DOI: 10.1021/acs.chemmater. 2c03787. |
| [53] | SOUNDHARRAJAN V, ALFAZA G, ARIFIADI A, et al. Na3.5(MnVFeTi)0.5(PO4)3: A multi-transition-metal-ion-engineered NASICON-type cathodes for sodium ion batteries[J]. Batteries & Supercaps, 2025, 8(3): e202400526. DOI: 10.1002/batt.202400526. |
| [54] | CUI G J, DONG Q Y, WANG Z Z, et al. Achieving highly reversible and fast sodium storage of Na4VMn(PO4)3/C-rGO composite with low-fraction rGO via spray-drying technique[J]. Nano Energy, 2021, 89: 106462. DOI: 10.1016/j.nanoen.2021. 106462. |
| [55] | GHOSH S, BARMAN N, MAZUMDER M, et al. High capacity and high-rate NASICON-Na3.75V1.25Mn0.75(PO4)3 cathode for Na-ion batteries via modulating electronic and crystal structures[J]. Advanced Energy Materials, 2020, 10(6): 1902918. DOI: 10.1002/aenm.201902918. |
| [56] | AHSAN M T, ALI Z, QIU D P, et al. A strategy to mitigate jahn teller effect of Mn-rich NASICON framework for sodium-ion batteries[J]. Small, 2024, 20(43): 2402275. DOI: 10.1002/smll. 202402275. |
| [57] | LI W, LI J P, LI R R, et al. Study on sodium storage properties of manganese-doped sodium vanadium phosphate cathode materials[J]. Battery Energy, 2023, 2(2): 20220042. DOI: 10.1002/bte2.20220042. |
| [58] | GHOSH S, BARMAN N, SENGUTTUVAN P. High capacity and high rate nasicon NaxV(Mn/Mg/Al)(PO4)3 cathodes for Na-ion batteries[J]. ECS Meeting Abstracts, 2021, MA2021-01(4): 255. DOI: 10.1149/ma2021-014255mtgabs. |
| [59] | XU C L, ZHAO J M, WANG E H, et al. A novel NASICON-typed Na4VMn0.5Fe0.5(PO4)3 cathode for high-performance Na-ion batteries[J]. Advanced Energy Materials, 2021, 11(22): 2100729. DOI: 10.1002/aenm.202100729. |
| [60] | YU S J, CHEN Y W, HUANG Y Y, et al. Na4MnCr(PO4)3 with a dual conductive network for sodium-ion batteries[J]. Advanced Sustainable Systems, 2025, 9(7): 2300233. DOI: 10.1002/adsu. 202300233. |
| [61] | LIU J H, WANG S, KAWAZOE Y, et al. Mechanisms of ionic diffusion and stability of the Na4MnCr(PO4)3 cathode[J]. ACS Materials Letters, 2022, 4(5): 860-867. DOI: 10.1021/acsmaterialslett. 2c00194. |
| [62] | CHEN Y, PENG P, SUN K Y, et al. Stabilizing NASICON-type Na4MnCr(PO4)3 by Ti-substitution toward a high-voltage cathode material for sodium ion batteries[J]. Journal of Colloid and Interface Science, 2024, 671: 385-393. DOI: 10.1016/j.jcis.2024. 05.130. |
| [63] | LI J, ZHAO X D, HE P G, et al. Stabilized multi-electron reactions in a high-energy Na4Mn0.9CrMg0.1(PO4)3 sodium-storage cathode enabled by the pinning effect[J]. Small, 2022, 18(31): 2202879. DOI: 10.1002/smll.202202879. |
| [64] | ZHU T, HU P, CAI C C, et al. Dual carbon decorated Na3MnTi(PO4)3: A high-energy-density cathode material for sodium-ion batteries[J]. Nano Energy, 2020, 70: 104548. DOI: 10.1016/j.nanoen.2020.104548. |
| [65] | QI Q, LI X D, LIANG J Q, et al. Effect of Zr4+ doping on the electrochemical properties of Na3MnTi(PO4)3/C cathode materials[J]. Journal of Electroanalytical Chemistry, 2023, 950: 117916. DOI: 10.1016/j.jelechem.2023.117916. |
| [66] | GAO H C, SEYMOUR I D, XIN S, et al. Na3MnZr(PO4)3: A high-voltage cathode for sodium batteries[J]. Journal of the American Chemical Society, 2018, 140(51): 18192-18199. DOI: 10.1021/jacs.8b11388. |
| [67] | MA X D, WU X H, LIU Y, et al. Toward a high-energy-density cathode with enhanced temperature adaptability for sodium-ion batteries: A case study of Na3MnZr(PO4)3 microspheres with embedded dual-carbon networks[J]. ACS Applied Materials & Interfaces, 2021, 13(18): 21390-21400. DOI: 10.1021/acsami. 1c03642. |
| [68] | PAREJIYA A, ESSEHLI R, AMIN R, et al. Na1+ xMnx/2Zr2- x/2(PO4)3 as a Li+ and Na+ super ion conductor for solid-state batteries[J]. ACS Energy Letters, 2021, 6(2): 429-436. DOI: 10.1021/acsenergylett. 0c02513. |
| [69] | ZHENG Y R, LIU J F, HUANG D, et al. Prepare and optimize NASICON-type Na4MnAl(PO4)3 as low cost cathode for sodium ion batteries[J]. Surfaces and Interfaces, 2022, 32: 102151. DOI: 10.1016/j.surfin.2022.102151. |
| [70] | ZHU Q, HU X L, TONG R, et al. Refining the electrochemical performance of Na4MnAl(PO4)3 cathode material by magnesium doping for sodium ion batteries[J]. Journal of Energy Storage, 2024, 92: 112258. DOI: 10.1016/j.est.2024.112258. |
| [71] | LIU Y, SUN C, LI Y, et al. Recent progress of Mn-based NASICON-type sodium ion cathodes[J]. Energy Storage Materials, 2023, 57: 69-80. DOI: 10.1016/j.ensm.2023.02.005. |
| [72] | DENG S P, SONG C R, LI S Y, et al. Rambutan-like Na4MnCr(PO4)3@C/CNTs as a high-energy-density cathode for sodium-ion batteries[J]. Solid State Ionics, 2024, 410: 116545. DOI: 10.1016/j.ssi.2024.116545. |
| [73] | LESLIE K, ABRAHAM J J, MACLENNAN H, et al. Reducing the rate of Mn dissolution in LiMn0.8Fe0.2PO4/graphite cells with mixed salt and low salt molarity electrolytes[J]. Journal of the Electrochemical Society, 2025, 172(4): 040515. DOI: 10.1149/1945-7111/adc951. |
| [74] | BENEDEK R. Role of disproportionation in the dissolution of Mn from lithium manganate spinel[J]. The Journal of Physical Chemistry C, 2017, 121(40): 22049-22053. DOI: 10.1021/acs.jpcc.7b05940. |
| [75] | FAN D S, WANG Y, ZHAO X D, et al. A novel NASICON-Na3.4MnV0.2Cr0.2Ti0.6(PO4)3 cathode with ultrahigh energy density and remarkable cycling stability toward practical Na-ion batteries[J]. Materials Today, 2025, 86: 63-73. DOI: 10.1016/j.mattod. 2025.03.010. |
| [76] | LU T M, SUN B X, DAI B H, et al. Efficient synthesis of NFPP sodium-ion battery cathode materials via a bimetallic iron source synergistic framework strategy[J]. Journal of Energy Storage, 2025, 107: 114976. DOI: 10.1016/j.est.2024.114976. |
| [77] | NGUYEN J, LEE Y, YANG Y. Suppression of high spin state of Mn for the improvement of Mn-based materials in rechargeable batteries[J]. Small, 2024, DOI: 10.1002/smll. 202410453. |
| [1] | Yuxi CHU, Chang MA, Hongguang CHEN, Shaoyu ZHANG, Ping ZHUO. Thermal runaway and gas production characteristics of a 180 Ah sodium-ion battery [J]. Energy Storage Science and Technology, 2025, 14(9): 3611-3618. |
| [2] | Chengshan XU, Han LI, Yan WANG, Languang LU, Xuning FENG, Minggao OUYANG. Research on fire propagation characteristics and energy transfer mechanisms during the triggering process in double-layer energy storage batteries [J]. Energy Storage Science and Technology, 2025, 14(9): 3552-3563. |
| [3] | Xinxin ZHANG, Guanjun CEN, Ronghan QIAO, Junfeng HAO, Qiangfu SUN, Bowen ZHENG, Yuhao GU, Mengyu TIAN, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Hong ZHOU, Xueji HUANG. Reviews of selected 100 recent papers for lithium batteries (June 1, 2025 to July 31, 2025) [J]. Energy Storage Science and Technology, 2025, 14(9): 3229-3248. |
| [4] | Haoyuan MA, Yan WU, Tong WANG, Jinyang HU, Jia LI, Yuqi HUANG. State of charge estimation of lithium iron phosphate batteries based on force-electric-temperature signals and a CNN-BiLSTM model [J]. Energy Storage Science and Technology, 2025, 14(7): 2865-2874. |
| [5] | Junfeng HAO, Jing ZHU, Guanjun CEN, Ronghan QIAO, Xinxin ZHANG, Qiangfu SUN, Mengyu TIAN, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Hong ZHOU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Apr. 1, 2025 to May 31 2025) [J]. Energy Storage Science and Technology, 2025, 14(7): 2884-2902. |
| [6] | Dandan HAN, Wuwei ZHANG, Liang ZHANG, Zongjiang WANG. Design and electrochemical performance of LiMn1-y Fe y PO4/C cathode materials with a core-shell structure [J]. Energy Storage Science and Technology, 2025, 14(6): 2215-2222. |
| [7] | Deshuai LIU, Huiqin ZHU, Ruihao SUN, Meng LI, Wenhao GONG, Xiaohui LI, Weiwei QIAN. Synergistic dual-additive boost cyclability of sodium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(5): 1858-1865. |
| [8] | Congqing TANG, Jingsheng CAI. Recent advances in presodiation strategies for sodium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(5): 1884-1899. |
| [9] | Qiangfu SUN, Guanjun CEN, Ronghan QIAO, Jing ZHU, Junfeng HAO, Xinxin ZHANG, Mengyu TIAN, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Hong ZHOU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Feb. 1, 2025 to March 31, 2025) [J]. Energy Storage Science and Technology, 2025, 14(5): 1727-1747. |
| [10] | Youwei WEN, Anqi TENG, Yongqi LI, Jiamin TIAN, Kangjie DING, Qiangling DUAN, Qingsong WANG. Electrical performance and heat production behavior of sodium-ion batteries at different discharge rate [J]. Energy Storage Science and Technology, 2025, 14(4): 1687-1697. |
| [11] | Yongqi LI, Zhiyuan LI, Youwei WEN, Chengdong WANG, Qiangling DUAN, Qingsong WANG. Experimental study of thermal runaway characteristics of large-capacity sodium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(4): 1657-1667. |
| [12] | Xinxin ZHANG, Guanjun CEN, Ronghan QIAO, Jing ZHU, Junfeng HAO, Qiangfu SUN, Mengyu TIAN, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Hong ZHOU, Xuejie HUANG. Reviews of 100 selected recent papers on lithium batteries (December 1, 2024 to January 31, 2025) [J]. Energy Storage Science and Technology, 2025, 14(3): 1310-1330. |
| [13] | Boyu LIU, Tengfei WANG, Qing PANG, Kaiyu CHEN, Hongyu WANG. Preparation and electrochemical performance of Mg-Cr co-doped LiNi0.5Mn1.5O4 cathode material [J]. Energy Storage Science and Technology, 2025, 14(3): 1097-1106. |
| [14] | Pengjie ZHU, Wei LI, Chu ZHANG, Hao SONG, Beibei LI, Xiumei LIU, Lili LIU. Study on early warning system for thermal runaway of lithium batteries in energy storage cabinets due to smoke and gas diffusion [J]. Energy Storage Science and Technology, 2025, 14(2): 624-635. |
| [15] | Jinhao YE, Junhui HOU, Zhengguo ZHANG, Ziye LING, Xiaoming FANG, Silin HUANG, Zhiwen XIAO. Thermal runaway characteristics and gas generation behavior of 100 Ah lithium iron phosphate pouch cell [J]. Energy Storage Science and Technology, 2025, 14(2): 636-647. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
