Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (6): 2278-2319.doi: 10.19799/j.cnki.2095-4239.2024.1256
• Energy Storage Materials and Devices • Previous Articles Next Articles
Gongrui WANG( ), Anping ZHANG, Xuanxuan REN, Mingzhe YANG, Yuning HAN, Zhongshuai WU(
), Anping ZHANG, Xuanxuan REN, Mingzhe YANG, Yuning HAN, Zhongshuai WU( )
)
Received:2024-12-29
Revised:2025-04-04
Online:2025-06-28
Published:2025-06-27
Contact:
Zhongshuai WU
E-mail:wanggongrui@dicp.ac.cn;wuzs@dicp.ac.cn
CLC Number:
Gongrui WANG, Anping ZHANG, Xuanxuan REN, Mingzhe YANG, Yuning HAN, Zhongshuai WU. High-voltage lithium cobalt oxide cathode: Key challenges, modification strategies and future prospectives[J]. Energy Storage Science and Technology, 2025, 14(6): 2278-2319.

Fig. 3
(a) Illustration of various intermediate phases of LCO (O1 and O3: “O”represents octahedral coordination environment of Li+ and the number represents the number of slabs of transition metal-oxygen (TMO2) in a unit cell; H1-3: representing the structure comprises alternating O1 and O3 blocks; O3(II) and O3(I): represent the TMO2 stacking type are preserved from O3 phase while the lithium/vacancy ordering are different from each other)[31]; (b) Structural variations for LCO in different delithiated states (dQdV, dQ: derivative quantity of electric charge, dV : derivative voltage)[22]; (c) Calculation of Li x CoO2 crystal parameters in various states (triangle, circle, pentagonal, diamond and square represent O3(I), O3(I), O3, H1-3 and O1 phase, respectively)[27]"
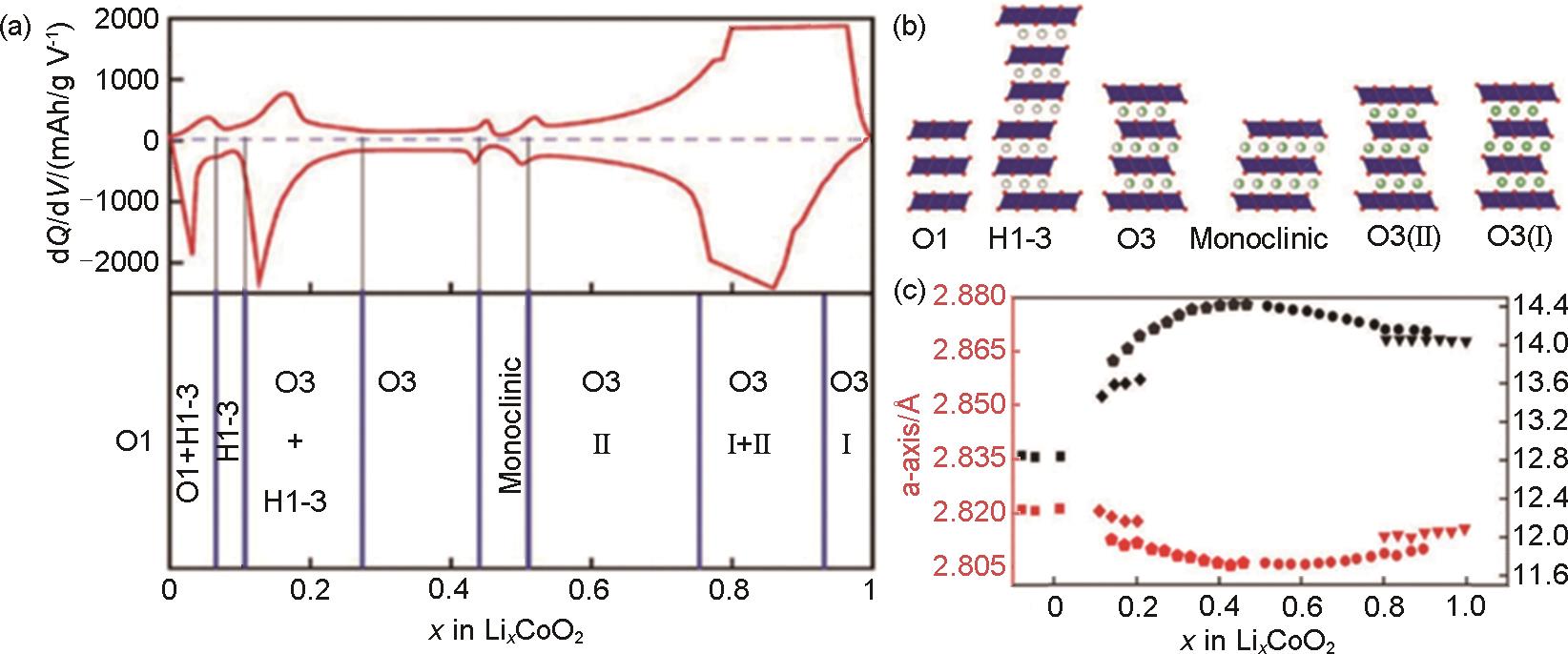

Fig. 4
(a) The O3-LiCoO2 (blue) transforms to O1-Li(1-x)CoO2 (orange) through the simultaneously consecutive glide in the ab plane and interlayer expanding along the c axis of CoO6 slabs[33]; (b) O3-LiCoO2 with δ1 = 9.847°[33]; (c) Charged Li0.5CoO2 and discharged Li0.65CoO2 with δ = 7.426° and 5.761°, respectively[33]; (d) O1-LiCoO2 with δ5 = 0°[34]; (e) Schematic illustration of the step-like surface degradation of high-voltage LCO[34]; TEM images for LCO (f) the first charge to 4.65 V, (g) after 200 cycles[35]; The reciprocal lattice pattern viewed along [210] direction for LCO (h) the first charge to 4.65 V; (i) the first discharge to 3 V, and (j) after 200 cycles[35]"
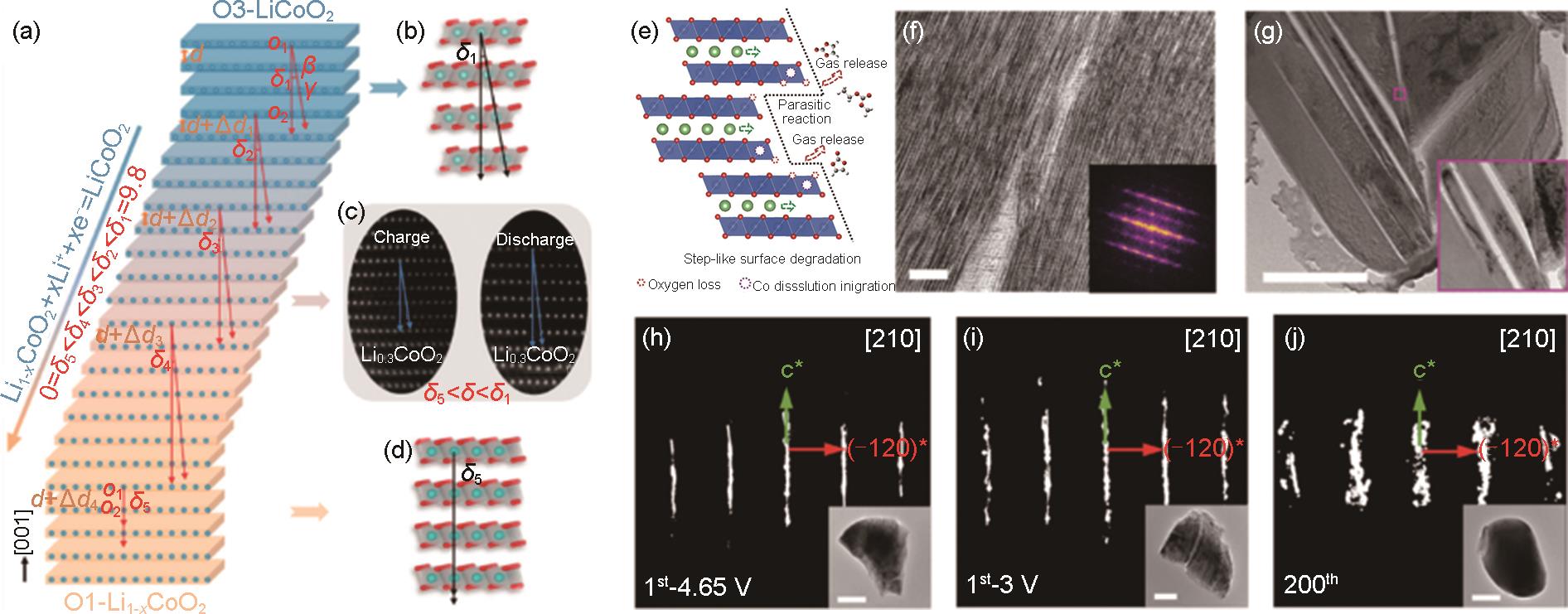
Fig. 5
Degradation mechanism of LCO-electrolyte interface at high voltage; (a) Possible interface failures: microcracks, loss of active material, phase transition, cobalt dissolution, oxygen release, gas production, electrolyte decomposition, CEI formation, HF attack, resistance increase, etc.[36]; (b) Degradation cycle based on HF attack and water regeneration[43]; (c) Surficial oxygen release causing structural transformation and electrolyte decomposition[46]"
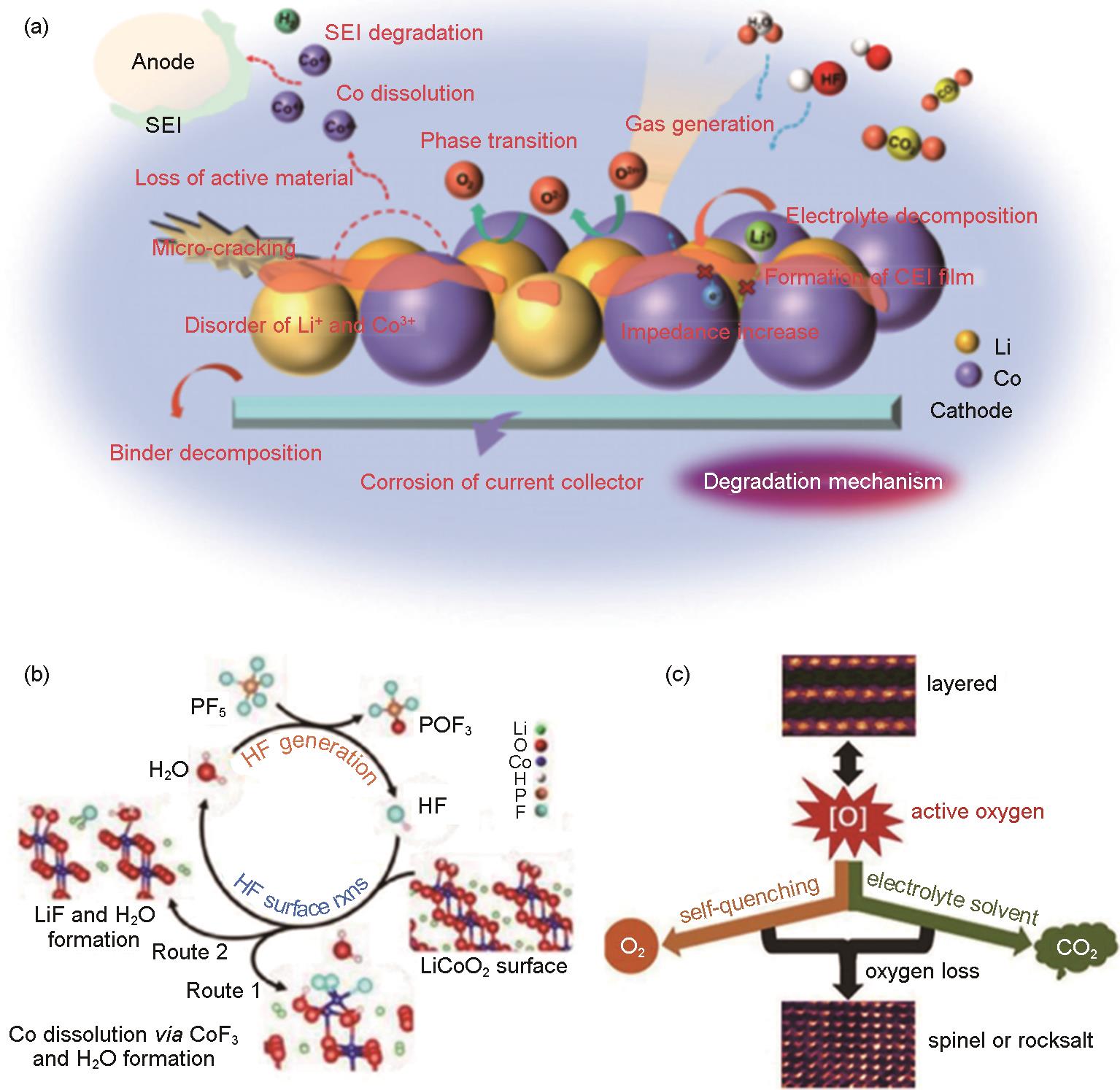

Fig. 6
Failure mechanism under high-voltage fast-charging conditions: (a)—(d) 3D and 2D Ni valence state distribution of (a), (c) particle and (b), (d) representative nanodomain for (a), (b) rod-NMC and (c), (d) gravel-NMC at charged state in the first cycle, The scale bars are 3 μm and 1 μm for(a), (c) and (b), (d), respectively[53]; Visualization of the particle detachment: (e) Selected slice in a NMC particle; (f) The 3D rendering of the segmentation results (left), the calculated distributions of relative local electrical resistance over the surface (medium), the same particles presented without the void phase (right); The scale bars are 10 μm in (e), (f)[55]; (g) Microtomography data of a piece of NMC electrode (left) and 3D rendering of the nano tomography data (right); (h)—(j) Three of the representative particles are highlighted in red, green, and blue; The same lateral slice on the particle layer (k) close to the top surface and (l) the bottom of ten-cycled electrode[58]; (m) Lithium composition maps of individual platelet particles at various SOC state during the second cycle[59]"
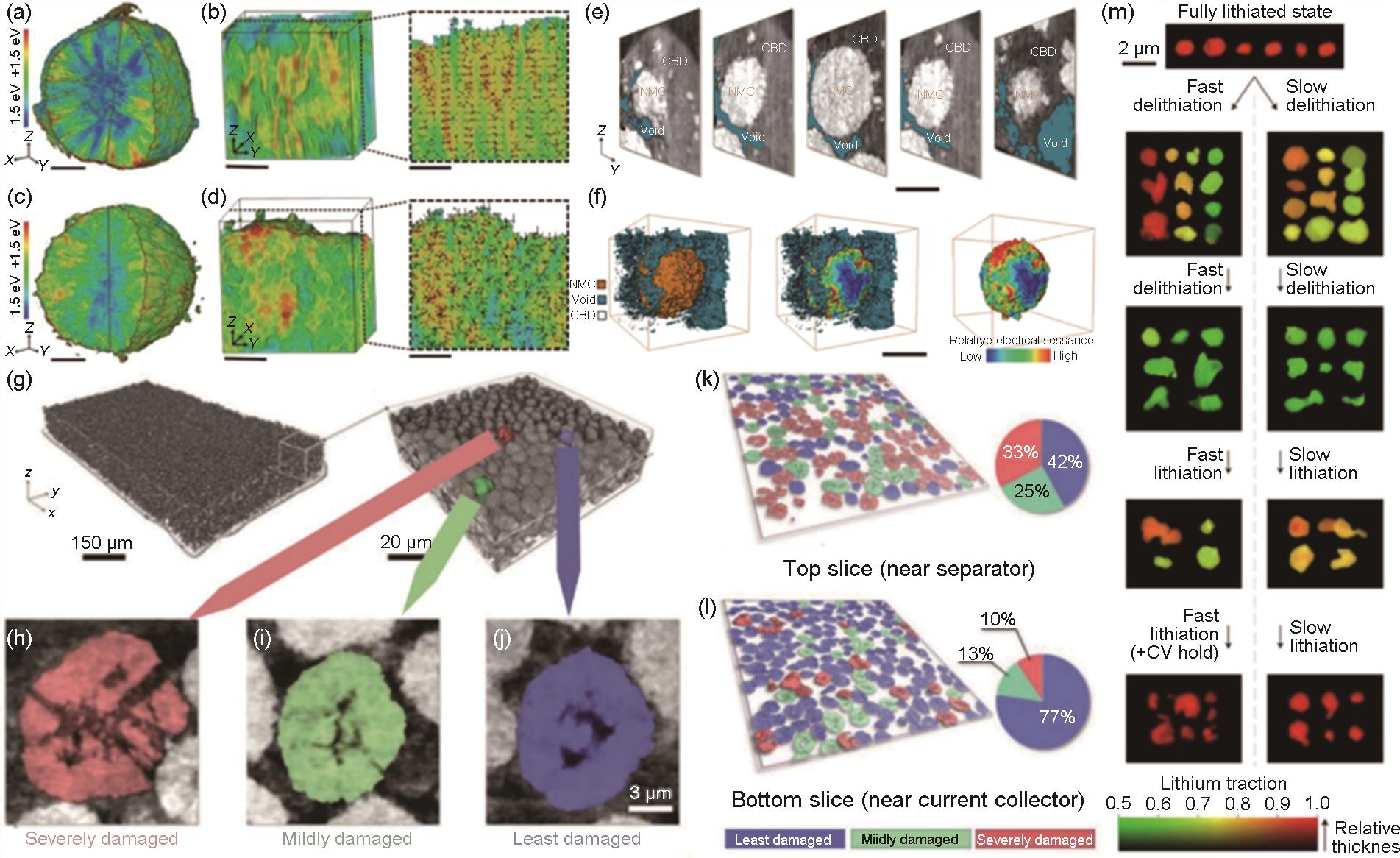
Fig. 7
TEM images of cathodes (a) before and after storing for (b) 1 month, (c) 3 months, and (d) 6 months; TEM images of anodes (e) before storage and after storing for (f) 1 month, (g) 3 months, and (h) 6 months at 65 ℃[60]; (i) Schematic illustration for the degradation mechanism of NCM811 electrode at elevated temperatures (80 ℃)[62]"
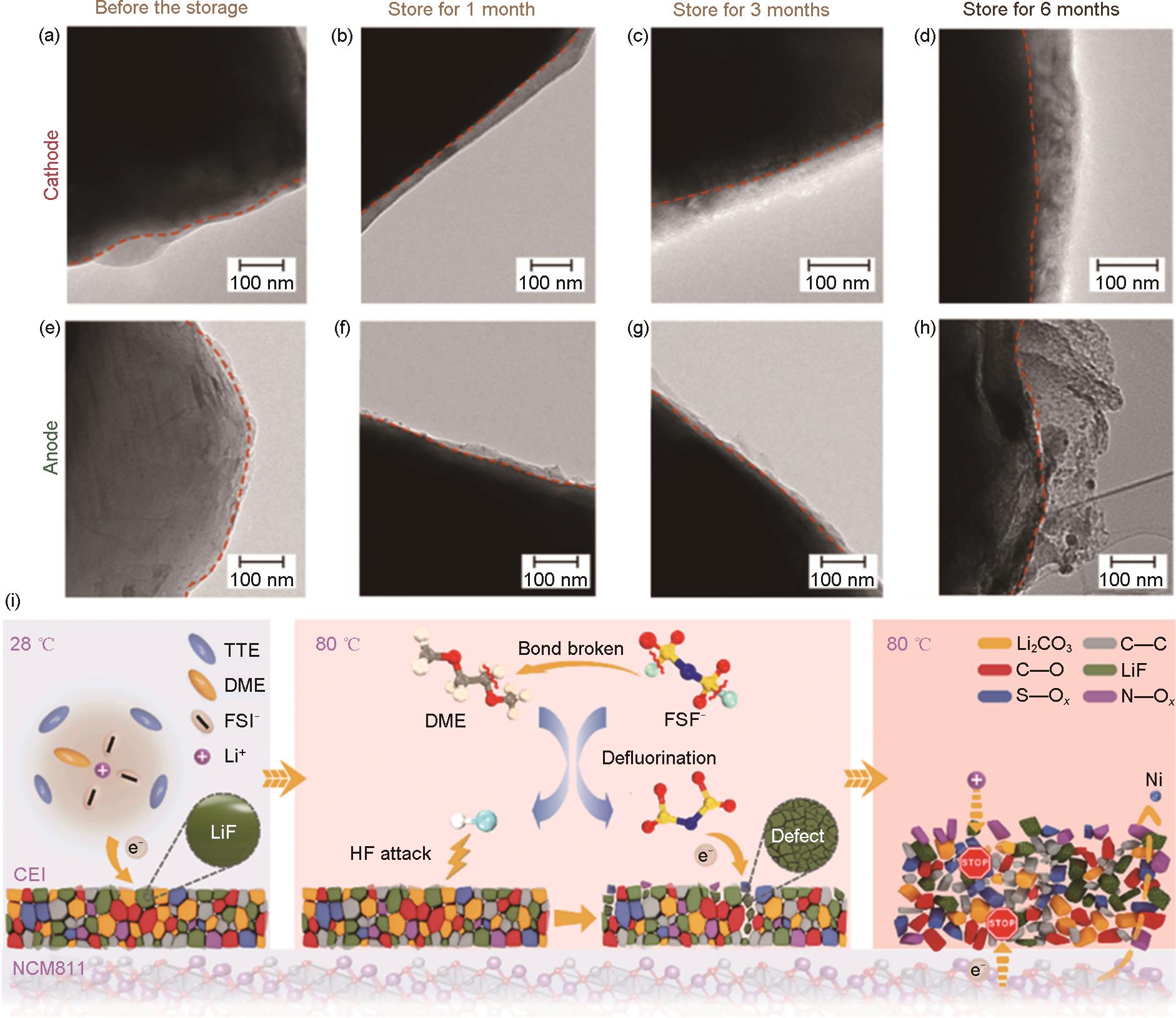
Table 1
Performance comparison of high-voltage fast-charging LCO modified with various dopants. EC∶ethylene carbonate, DEC∶diethyl carbonate, DMC∶dimethyl carbonate, EMC∶ethyl methyl carbonate, FEC∶fluoroethylene carbonate, VC∶vinylene carbonate"
| Type | Dopants | Electrolyte | Mass loading /(mg/cm2) | Cutoff voltage (vs. Li+/Li)/V | Cyclability (capacity retention-ycles-rates) | Rate capability/(mAh/g-rates) | Refs. |
|---|---|---|---|---|---|---|---|
| lithium ion site | Mg | 1 mol/L LiPF6 in EC/DEC | ~3 | 4.6 | 84-100-1C | 138-4C | [ |
| Ni | 1 mol/L LiPF6 in EC/DMC/DEC | ~15.2 | 4.45 | 93%-100-1C | 150-5C | [ | |
| Cu | 1 mol/L LiPF6 in EC/DEC | — | 4.4 | 89%-500-1C | 144-20C | [ | |
| W | 1 mol/L LiPF6 in EC/DMC | — | 4.6 | 72%-100-0.1C | — | [ | |
| (SO4) n- | 1 mol/L LiPF6 in EC/EMC/DMC with 5% VC | ~2 | 4.6 | 88%-100-1C | 143-20C | [ | |
| cobalt ion site | Al | 1 mol/L LiPF6 in EC/DEC/DMC | ~3 | 4.6 | 94%-30-0.1C | — | [ |
| V | 1 mol/L LiPF6 in EC/DMC | ~2 | 4.6 | 84%-100-1C93%-200-5C | 80-5C | [ | |
| Zr | 1 mol/L LiPF6 in EC/EMC/DMC | — | 4.5 | 75%-50-1C | 100-3C | [ | |
| oxygen io site | Se | 1.2 mol/L LiPF6 in EC/DEC | ~12 | 4.62 | 86%-120-0.25C | — | [ |
| multiple sites | Al, La | 1.2 mol/L LiPF6 in EC/EMC | ~10 | 4.5 | 96%-50-0.1C | 165-2C | [ |
| Al, F | 1 mol/L LiPF6 in EC/DMC | ~3 | 4.6 | 86%-200-1C | 108-10C | [ | |
| Mg, F | 1 mol/L LiPF6 in EC/DMC | ~3 | 4.6 | 92%-100-1C84%-1000-5C | 140-5C | [ | |
| Mg, manganese (Mn) | 1 mol/L LiPF6 in EC/DEC | — | 4.7 | 30%-50-0.2C | — | [ | |
| Ti, Mg, Ni | 1 mol/L LiPF6 in EC/EMC/DMC | ~1.8 | 4.5 | 90%-100-1C | 137-5C | [ | |
| Nb, W, Al | 1 mol/L LiPF6 in EC/EMC with 10% FEC | ~5 | 4.5 | 60%-1000-10C | 142-15C | [ | |
| Ti, Mg, Al | 1 mol/L LiPF6 in EC/EMC with 2% VC | ~4 | 4.5 | 97%-100-0.33C | 165-20C | [ | |

Fig. 8
(a) Co k-edge XAFS after Fourier transform; (b) CoO4 plane and Co-O bond length of C-LCO (top) and P-LCO (bottom). Calculated PDOS and corresponding Co 3d: t2g and O 2p bandgap plots for (c) C-LCO and (d) P-LCO; (e) Diagram of electron orbital occupancy changes; (f) Co spin configuration differential plots of C-LCO and P-LCO; (g) Changes in the contents of C-LCO and (h) P-LCO in each phase around 4.6 V. Schematic diagram of the structure of the O3 phase and the H1-3 phase[81]; (i) Fine crystal structure of NH4VO3-treated LCOs (denoted as NV-30LCO) for the first cycle; (j) Differential electrochemical mass spectrometry of NV-30LCO and (k) blank LCOs (denoted Bare-LCO); (l) Schematic diagram of the calculated Bader charge and V-doping of CoO6/VO6[71]"
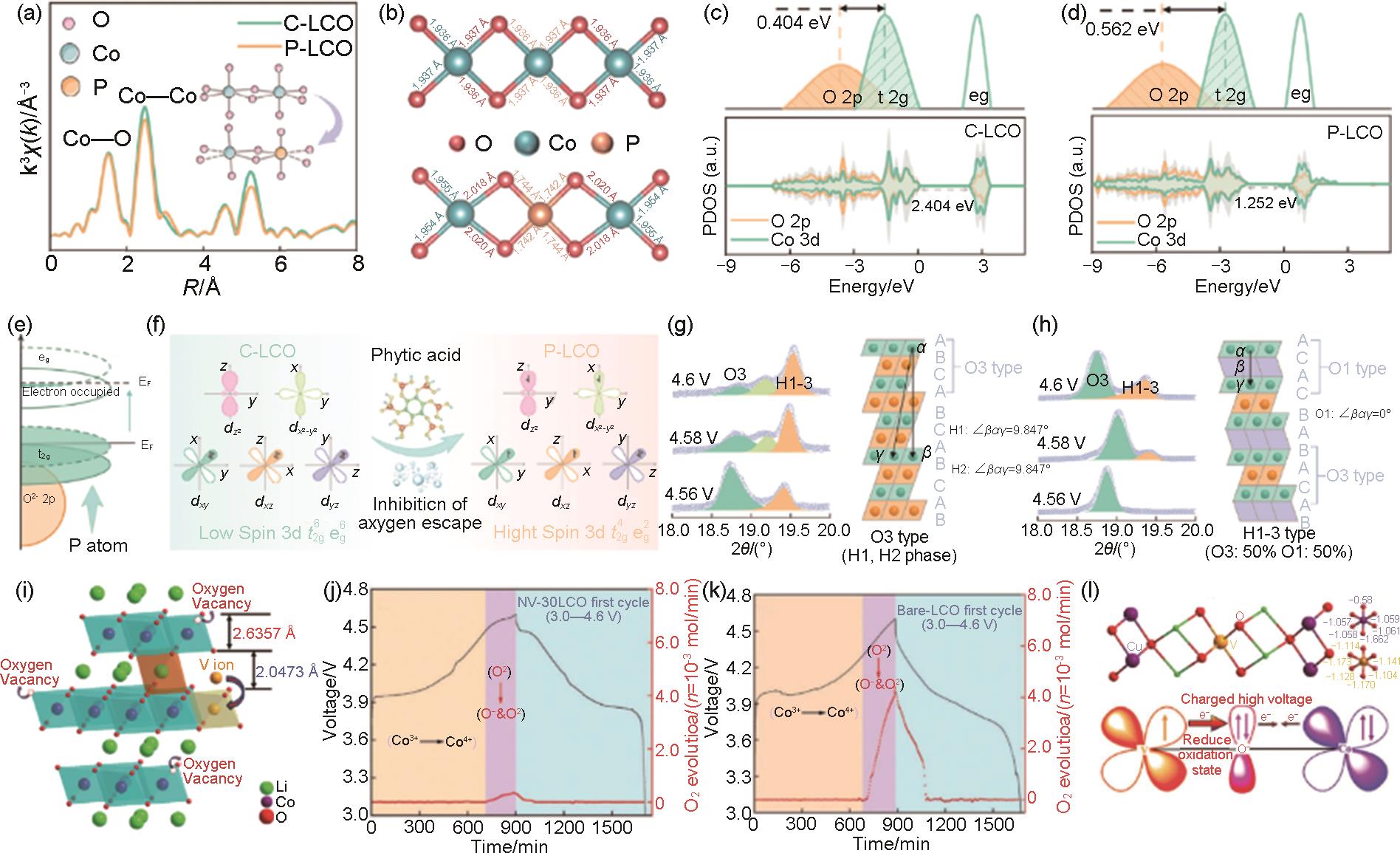
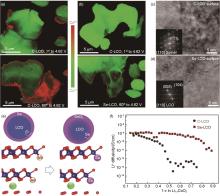
Fig. 9
Co valence mapping in (a) C-LCO and (b) Se-LCO in the 1st and 60th cycles at a charged state (4.62 V); (c), (d) HRTEM images of the superficial layer of (c) C-LCO and (d) Se-LCO at the 120th cycle (the inset shows the corresponding fast Fourier transform (FFT) patterns); 1st: the first cycle; (e) Schematic and lattice structure of (left) mobile O escaping from the charged LCO lattice and (right) Se substituting for Ov; (f) Diffusion coefficients of Li+ at the 120th cycle of C-LCO and Se-LCO, respectively[98]"
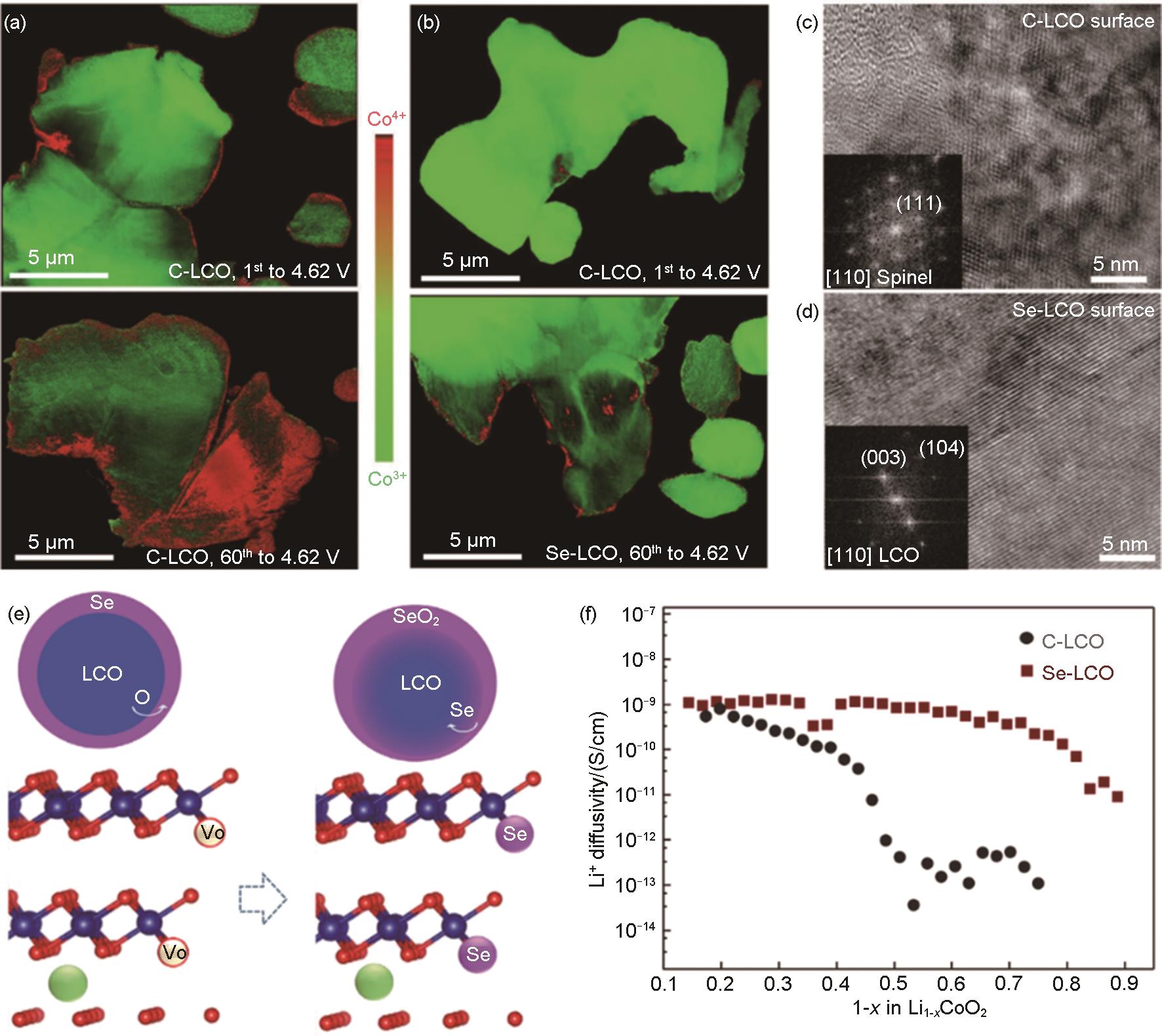

Fig. 10
(a) Formation energies of LCO and Mg-doped LCO with different doping sites; (b) Structural evolution illustration between LCO and LMCO; (c) STEM-HAADF and (d) STEM-ABF images of LMCO; (e) Path 1 belongs to the neighboring Li sites along a/b-axis direction, (g) Path 2 along the diagonal neighboring Li ions. The corresponding Li ions migration energy of LCO and LMCO; (f) Path 1 and (h) Path 2[99]; (i) Schematic diagram of the function of substituted nickel ions in the lithium vacancies, the “screen effect”[101]; (j) High-resolution STEM images of the layered structure at the center (left) and surface (right); (k) Schematic figure of the ion exchanging mechanism; (l) The rate capability at 0.1C charging and discharging at various current densities[67]"
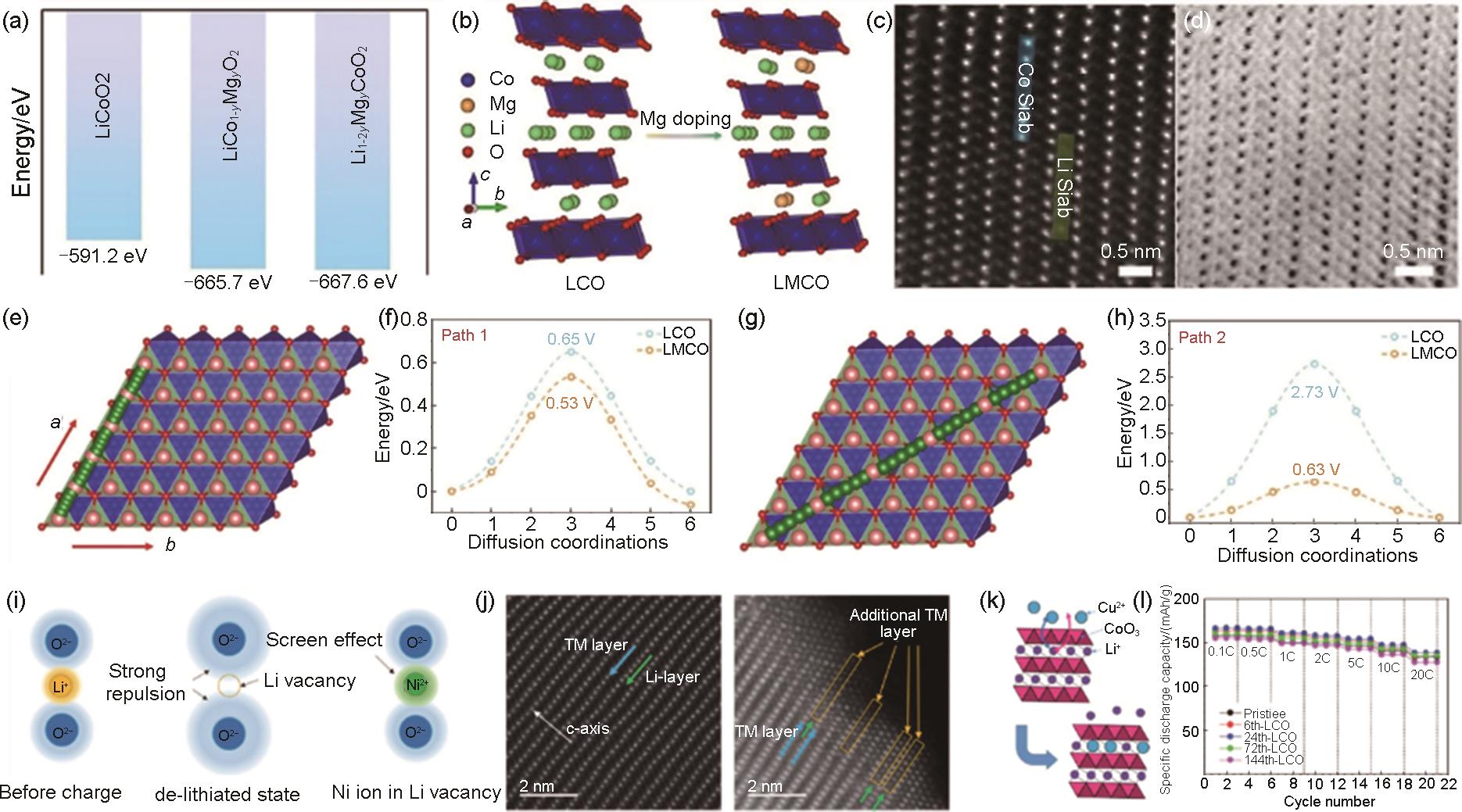
Fig. 11
Schematic of crystal structure at the surface of (a) N@P and (b) Pristine (denoted as Pri); HAADF-STEM images of (c) N@P (left) and Pri (right); (d, e) The calculated Li diffusion coefficient obtained from GITT for Pri and N@P; (f) Rate capability of Pri and N@P; (g) Cycle performance of N@P in a pouch cell over 3.0—4.6 V[105]; (h) Raman spectra of pristine LCO and phosphidated LCO; (i) O 1s XPS spectra of phosphidated LCO; F 1s XPS spectra for (j) pristine LCO and (k) phosphidated LCO upon initial cycle[106]; (l) Schematic illustration of the synthesis of coherent spinel Li x Co2O4 and Li2SO4 co-coated and high-valence S gradiently doped LCO; (m) Atomic-resolution HAADF-STEM images of EG-LCO-20+0.1S; (n) Rate performance of EG@-LCO-20, EG-LCO-20 and EG-LCO-20+0.1S[104]"
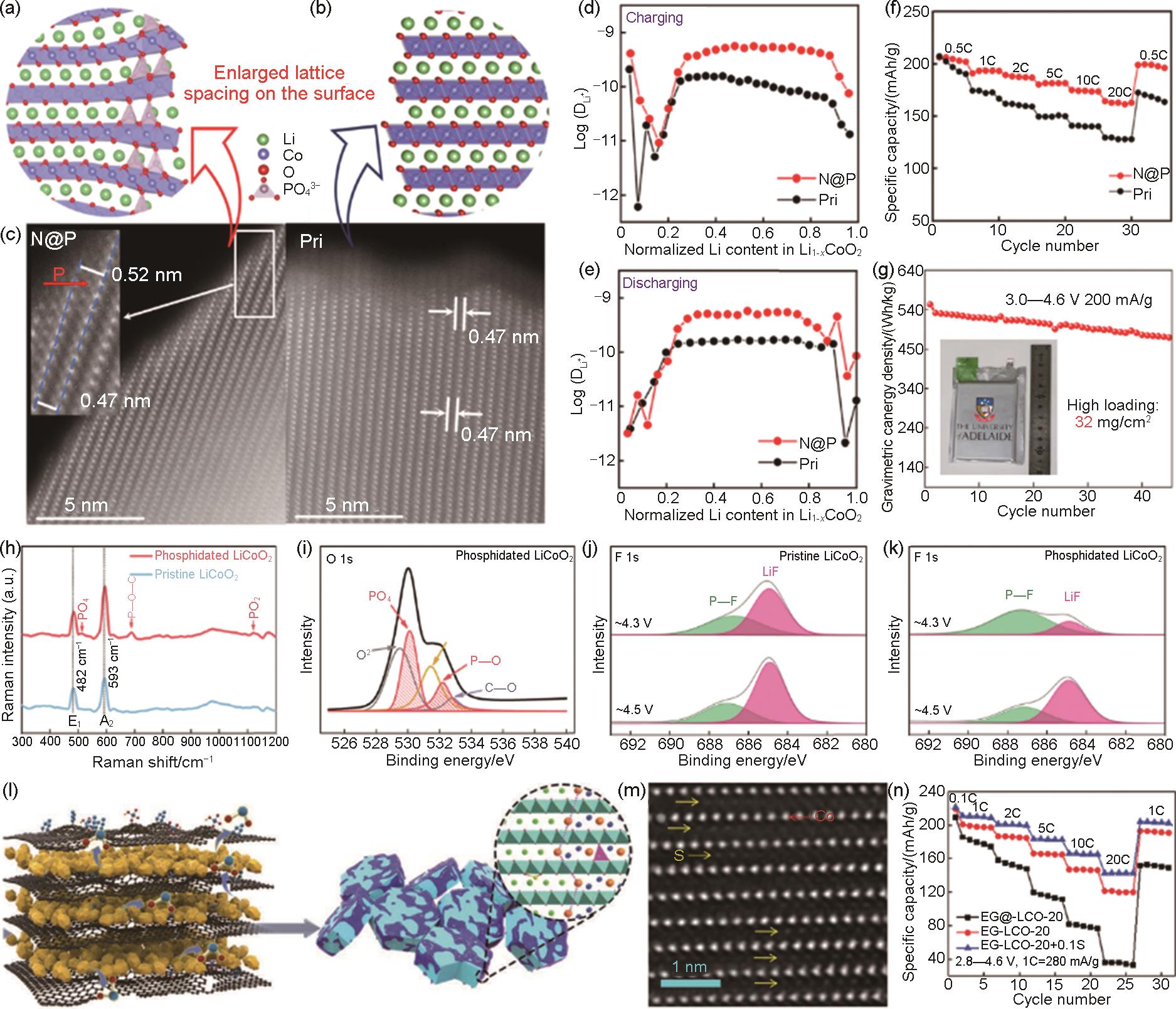
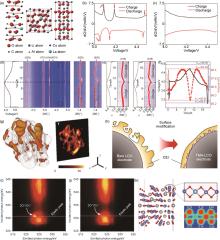
Fig. 12
(a) Schematic structures of doped CoCO3, Co3O4 and D-LCO; The dQ/dV curves of (b) P-LCO and (c) D-LCO (the red arrows refers to various phase transitions); (d) The voltage profile corresponded contour plot of XRD pattern evolution of D-LCO (the black dash circles refers to O3(I)-O3(II) transition), the (003) peaks evolution of D-LCO and P-LCO; (e) The contour plot of (015) peaks evolution of D-LCO and P-LCO; (f) The voltage profile (black) and the cell volume evolution process (red) of D-LCO; (g) 3D spatial distributions and elemental distributions of Ti[74]; (h) Schematic of the differences in CEI between bare LCO and TMA-LCO. O K edge RIXS maps of (i) bare LCO and (j) TMA-LCO at 4.6 V charged state; (k) Optimized atomic structures of the Ti-doped slab in Li0.29CoO2[37]"
Table 2
Performance comparison of high-voltage fast-charging LCO modified with various coating materials"
| Type | Coating layer | Electrolyte | Mass loading/(mg/cm2) | Cutoff voltage (vs. Li/Li+)/V | Cyclability (capacity retention-cycles-rate) | Rate capbility/(mAh/g-rates) | Refs. |
|---|---|---|---|---|---|---|---|
| dielectric materials | Al2O3 | 1 mol/L LiPF6 in EC/DMC/EMC | — | 4.5 | 71%-200-0.1C | — | [ |
| Al2O3 | 1 mol/L LiPF6 in EC/DEC/DMC | ~2 | 4.6 | 82.6%-500-1C | 100-10C | [ | |
| Al2O3 | 1 mol/L LiPF6 in EC/EMC | ~2 | 4.6 | 88%-200-0.5C | 165-3C | [ | |
| BaTiO3 | 1 mol/L LiPF6 in EC/DEC | — | 4.2 | 90%-800-5C | 60-100C | [ | |
| Al-Zn-O | 1 mol/L LiPF6 in EC/DEC | — | 4.6 | 65%-500-1C | 80-10C | [ | |
| MgO | 1 mol/L LiClO4 in PC | — | 4.4 | — | — | [ | |
| AlF | 1 mol/L LiPF6 in EC/DEC | — | 4.6 | 80%-50-0.5C | — | [ | |
| ionic conductive materials | spinel LiCo x Mn2-x O4 | 1 mol/L LiPF6 in EC/DMC | — | 4.5 | 82%-300-0.2C | 143-10C | [ |
| spinel LiCo2O4 | 1 mol/L LiPF6 in EC/EMC/DMC | ~2 | 4.6 | 97%-100-1C | 139-20C | [ | |
| spinel LiNi0.5Mn1.5O4 | 1.15 mol/L LiPF6 in EC/DEC with 5% FEC, 3% 1,3-propane sultone and 2% adiponitrile | ~2.5 | 4.45 | 81%-400-0.5C | — | [ | |
| LiAlF | 1 mol/L LiPF6 in EC/DEC | ~12.6 | 4.6 | 81%-200-0.1C | 100-3C | [ | |
| LiAlF | 1 mol/L LiPF6 in EC/DMC | — | 4.6 | 78%-500-0.5C | 125-10C | [ | |
| LiAlO2 | 1 mol/L LiPF6 in EC/DEC | ~15 | 4.6 | 83%-50-0.25C | 100-0.5C | [ | |
| LiF | 1 mol/L LiPF6 in EC/EMC/DMC | — | 4.2 | 77%-100-1C | — | [ | |
| LiAlH4 | 1 mol/L LiPF6 in EC/DEC/FEC | ~6 | 4.6 | 71%-1000-1C | 130-10C | [ | |
| LiCoPO4 | 1 mol/L LiPF6 in EC/EMC with 5% FEC | ~3 | 4.6/4.7 | 87%-300-1C | 178-10C | [ | |
| Li1.5Al0.5Ge1.5(PO4)3 | 1.2 M LiPF6 in EC/EMC | ~9 | 4.5 | 88%-400-0.3C | 163-6C | [ | |
| LiZr2(PO4)3 | 1 mol/L LiPF6 in EC/EMC/DMC | ~1.5 | 4.6 | 75%-100-0.5C | 75-5C | [ | |
| Li1.5Al0.5Ti1.5(PO4)3 | 1 mol/L LiPF6 in EC/DMC | ~3 | 4.6 | 88%-100-0.5C | 100-5C | [ | |
| electronic conductive materials | graphene | 1 mol/L LiPF6 in EC/DMC | — | 4.2 | 85%-200-1C | 100-5C | [ |
| multiple materials | AlPO4, Li3PO4 | 1 mol/L LiPF6 in EC/DEC | ~3 | 4.6 | 88%-200-0.5C | 149-5C | [ |
| Li1.5Al0.5Ge1.5P3O12, Al-doped ZnO | 1 mol/L LiPF6 in EC/DMC | ~2 | 4.6 | 77%-350-1C | 139-10C | [ | |
| LiF and Li2Co Ti3O8 | 1 mol/L LiPF6 in EC/DEC | ~8 | 4.6 | 81%-200-0.1C | 130-3C | [ | |
| Li2SrSiO4 and Al2O3 | 1 mol/L LiPF6 in EC/DEC | ~3 | 4.5 | 64%-500-10C | 80-10C | [ | |
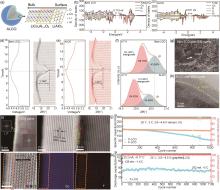
Fig. 13
(a) Schematic diagram of ALCO with multilayer surface structure composed of LiAlO2 and LiCo x Al1-x O2; PDOS values of selected and total elements of (b) bare LCO and (c) ALCO. In-situ XRD plot and corresponding charge/discharge curve at 0.1C for (d) bare LCO and (e) ALCO; (f) The XRD plot at 4.7 V charged state for bare LCO and ALCO; (g) SEM images of bare LCO and (h) TEM images of ALCO after 200 cycles[139]; (i) Characterization and compositional analysis of F-LCO fluorinated interfacial structure; (j) cycling performance of F-LCO and P-LCO; (k) cycling performance of Graphite||F-LCO pouch cells[140]"
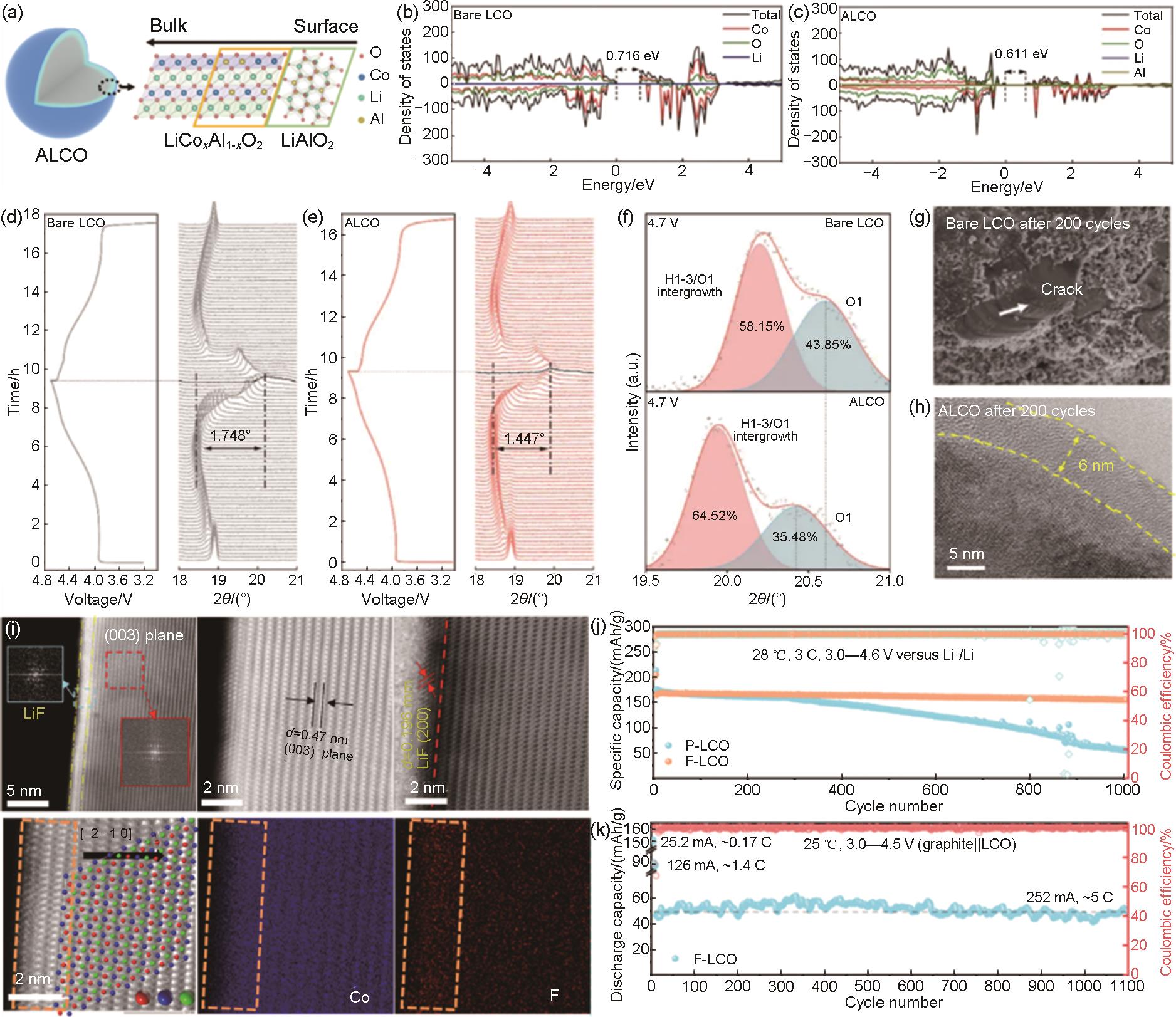
Fig. 14
(a) SEM images of LCO particles and carbon black in R-LCO; (b) Scheme of electrochemical indentation and crack formation in LCO crystals during charging R-LCO to a high voltage; (c) SEM images of CNT-cocooned LCO particles in CNT-LCO; (d) Scheme for preventing electrochemical indentation and crack formation in the LCO crystal when charging CNT-LCO to a high voltage; (e) Li concentration in the LCO particle in R-LCO; (f) the von Mises stress field of LCO in R-LCO; (g) Li concentration in the LCO particle in CNT-LCO; (h) the von Mises stress field of LCO in CNT-LCO; (i) Images of the free-standing CNT-LCO electrode before pressing. The inset shows the LCO particles in CNT-LCO; (j) Images of the free-standing CNT-LCO electrode after rolling pressing to a high density[153]"
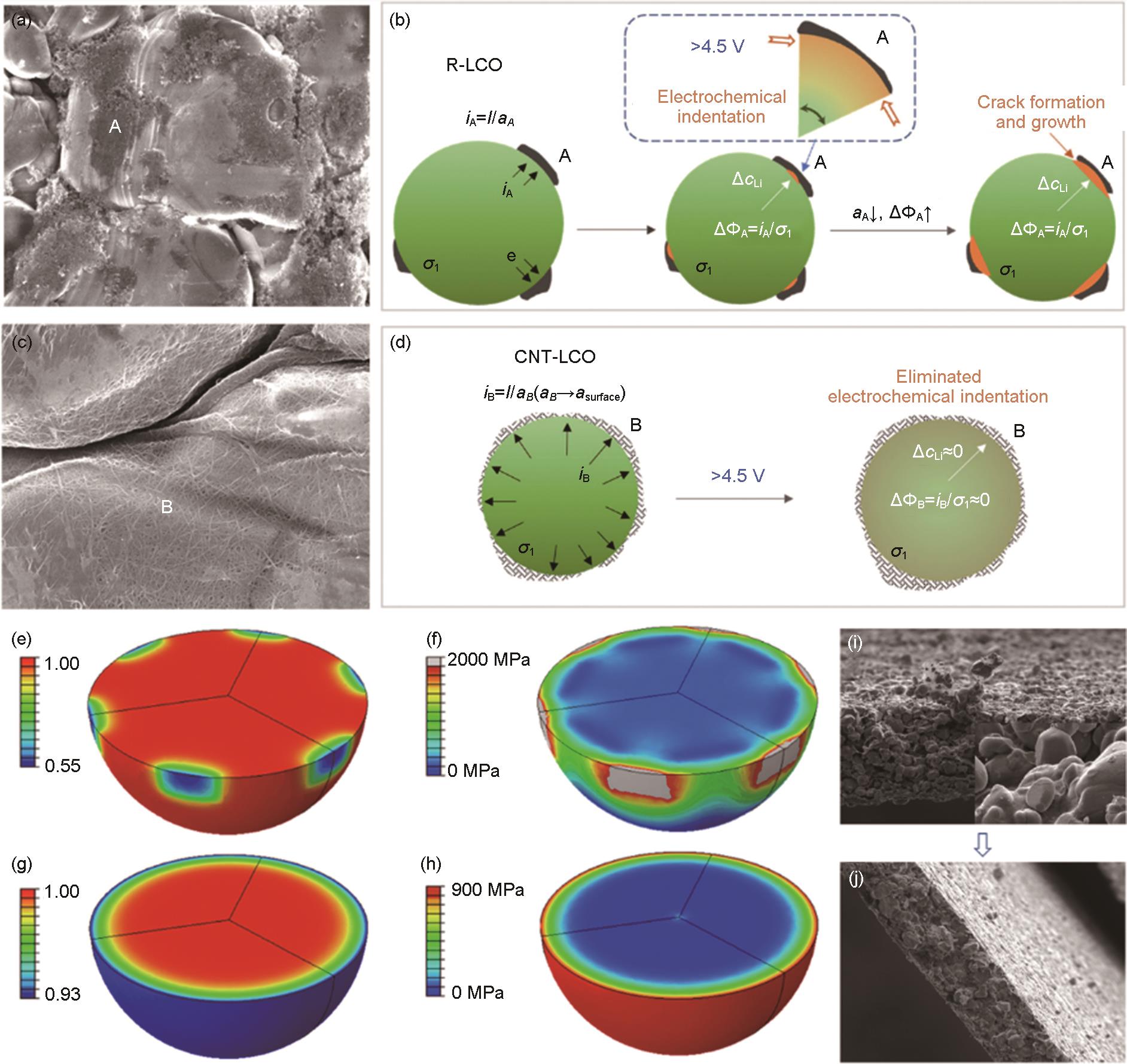
Fig. 15
(a) The adsorb energy of the cathode and different ingredients at the interface of pristine LCO (P-LCO) and Y-LCO; Microstructures and related FFT patterns (inset) of different CEI for (b) P-LCO and (c) Y-LCO after 100 cycles; The energy diagram of the decomposition of LiPF6 to PF5 and LiF on the surface of (d) delithiated LCO (donated as CoO2) and (e) Y2O3; (f) The Ab initio molecular dynamics (AIMD) simulation of LiPF6 on Y2O3 with different times and (g) the related electron localization function[154]; (h) HAADF-STEM image and illustration of the interphase for AlZnO-LCO; (i) Electrochemical impedance spectra (EIS) for various electrodes measured before/after cycling[120]"
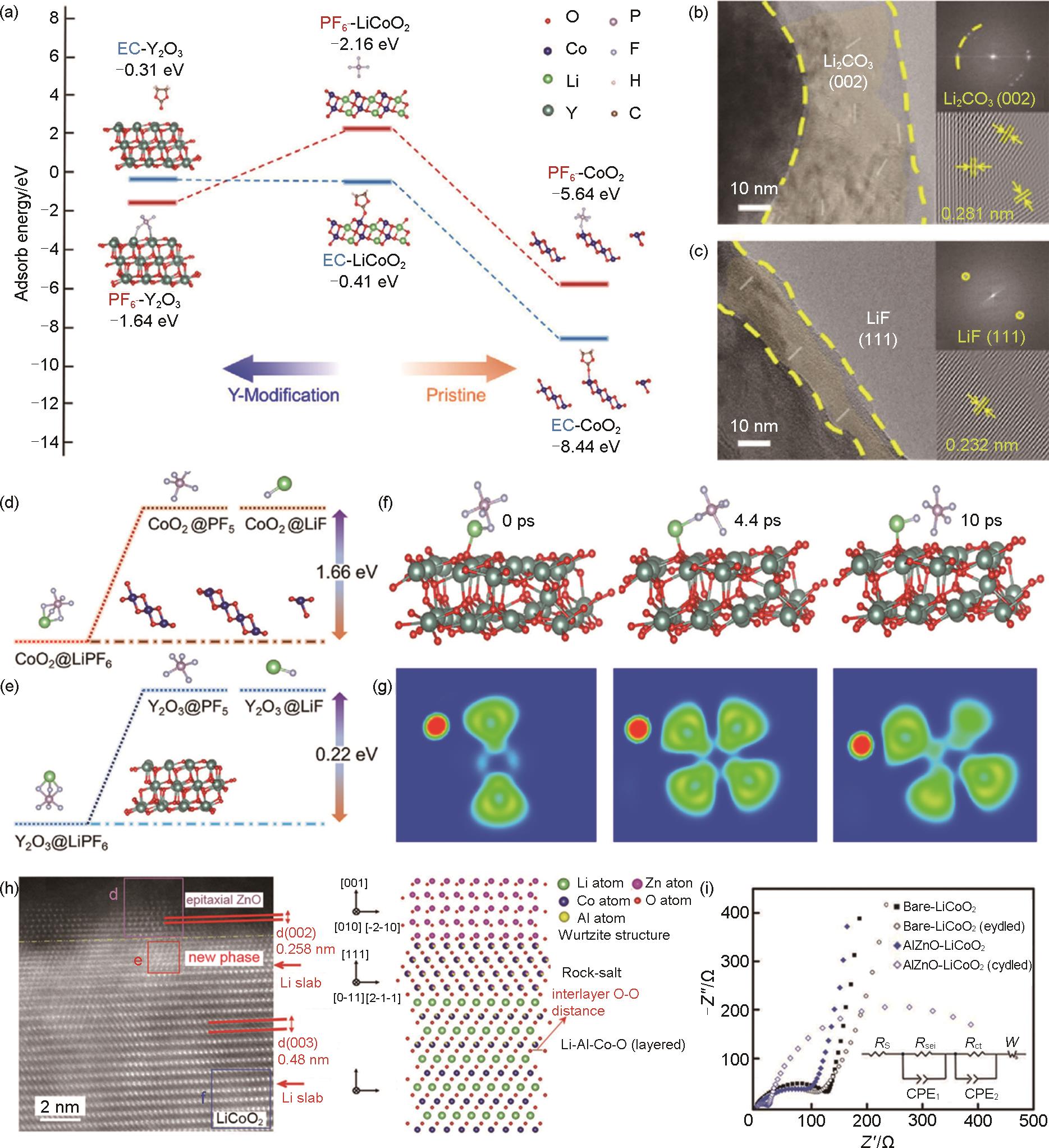
Fig. 16
(a) Schematic illustration of surface architecture and chemical potential profile μ of La3+ and Ca2+; δ denotes the oxygen off-stoichiometry; (b) Schematic of the atomic structure; (c) HAADF-STEM of La-LCO surface (after heat treatment) showing an architecture consisting of Region I, II and III, separated by the dashed lines, Scale bare 2 nm; (d) HAADF-STEM images and corresponding FFT patterns of Region I (up, perovskite La1-w Ca w CoO3-δ ) and III (down), Scale bar 5 nm; (e) PDOS of O 2p states and the local schematic structures of oxygen (insets) of LCO (left), unstrained LaCoO3 (medium), and -8% strained LaCoO3 (right). EF is the Fermi level, and solid lines are the average energy of the O 2pstates; (f) Characterization of the depth-dependent electrochemistry[156]; (g) Morphology of surface Li3PO4; (h) Surface, subsurface structures, and diffraction pattern analyses; (i) Schematic illustration of surface structure of SE-LCO; (j) Comparison of rate performances; (k) TEM image of the step-like surface degradation for P-LCO, and (l) the corresponded surface structures and related diffraction pattern analyses; (m) TEM image of the smooth surface for SE-LCO, and (n) the corresponded surface structures and related diffraction pattern analyses[33]"
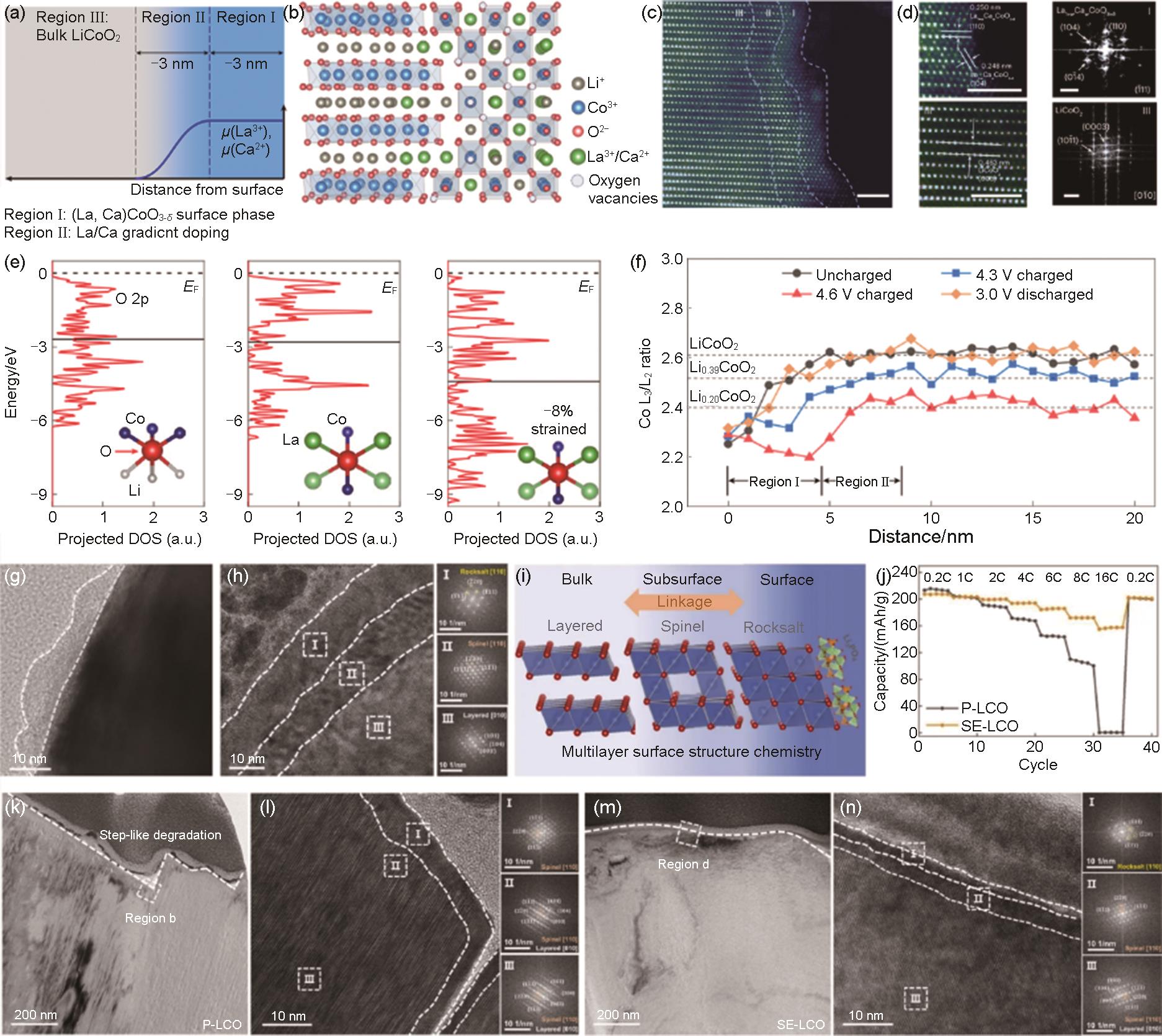
Fig. 17
(a) Schematic diagram of the GDLCO particle with gradient Li/Co disorder structure; (b) The calculated energy as a function of the strain along the c axis for ordered and disordered samples at fully delithiated states; (c) HRTEM image for GDLCO; X-ray fluorescence spectroscopy (XRF) images (left) and spatial visualizations (right) of lattice expansion/contraction (Δd/d) for (d) LCO and (e) GDLCO[159]; (f) The 3D Co valence distribution in a single particle of P-LCO (left) and LCO (right) cathode; (g) Stress distribution of P-LCO and LCO cathode particles during the charging process using finite-element simulation[160]"
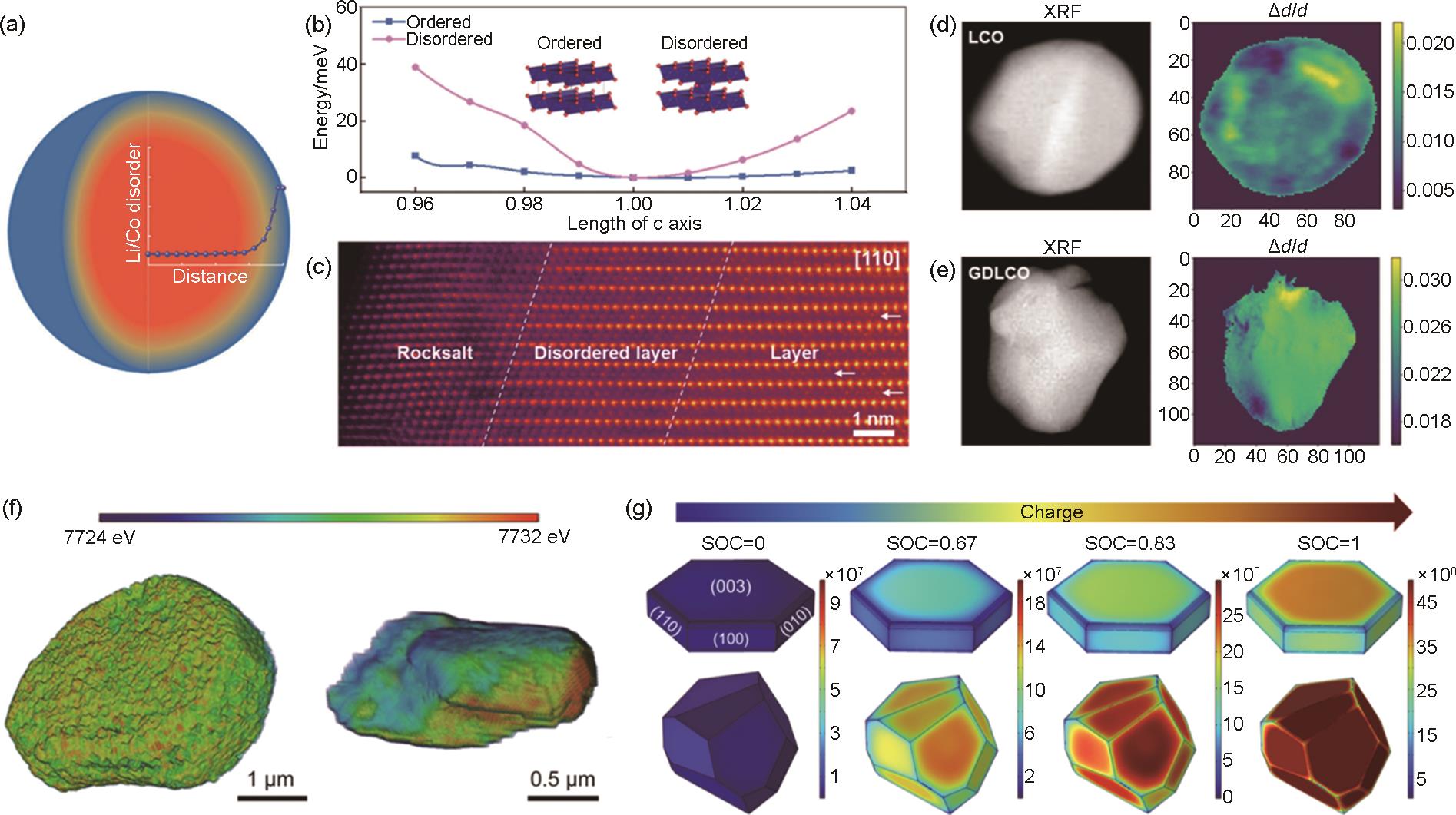
Fig. 18
(a) In-situ ATR-FTIR spectra of the LCO/electrolyte interface collected during initial charging; The current density is 25 mA/g; The spectrum collected at inflection voltage is colored in red. Schematic illustration of the electrolyte solvation configuration at the LCO/electrolyte interface during the Li+-solvation (charging) process at different voltages; (c) TOF-SIMS characterization of the 4.1 V and 4.2 V cycled LCO cathodes (below/above the inflection voltage) retrieved from the coin cells after 5 cycles. Normalized depth profiles of representative inorganic and organic fragments illustrate the CEI architecture; (d) Schematic illustration of CEI structure formed at charge cut-off voltages below/above inflection voltage[162]; Proposed functional mechanism of SPTF additive in carbonate electrolyte. HRTEM images of cycled LCO in base (e) and SPTF (f) electrolyte; (g) Electrochemical performance of LCO||graphite pouch cell[163]; (h) Schematic illustration of the mechanism on the stabilized 4.6 V LCO batteries; (i) Selected region of the (003) plane from the ex situ XRD patterns of LCO electrode cycled in FPE (up) and in TCE (down) during the 4th charging-discharging process at 0.2 C; (j) Long-term cycling performance of Gr||LCO coin cells obtained at 3C at 3—4.55 V (equivalent to 4.6 V vs. Li+/Li); (k) long-term cyclability of Gr||LCO pouch cells measured at 290 mA at 3—4.55 V[164]"
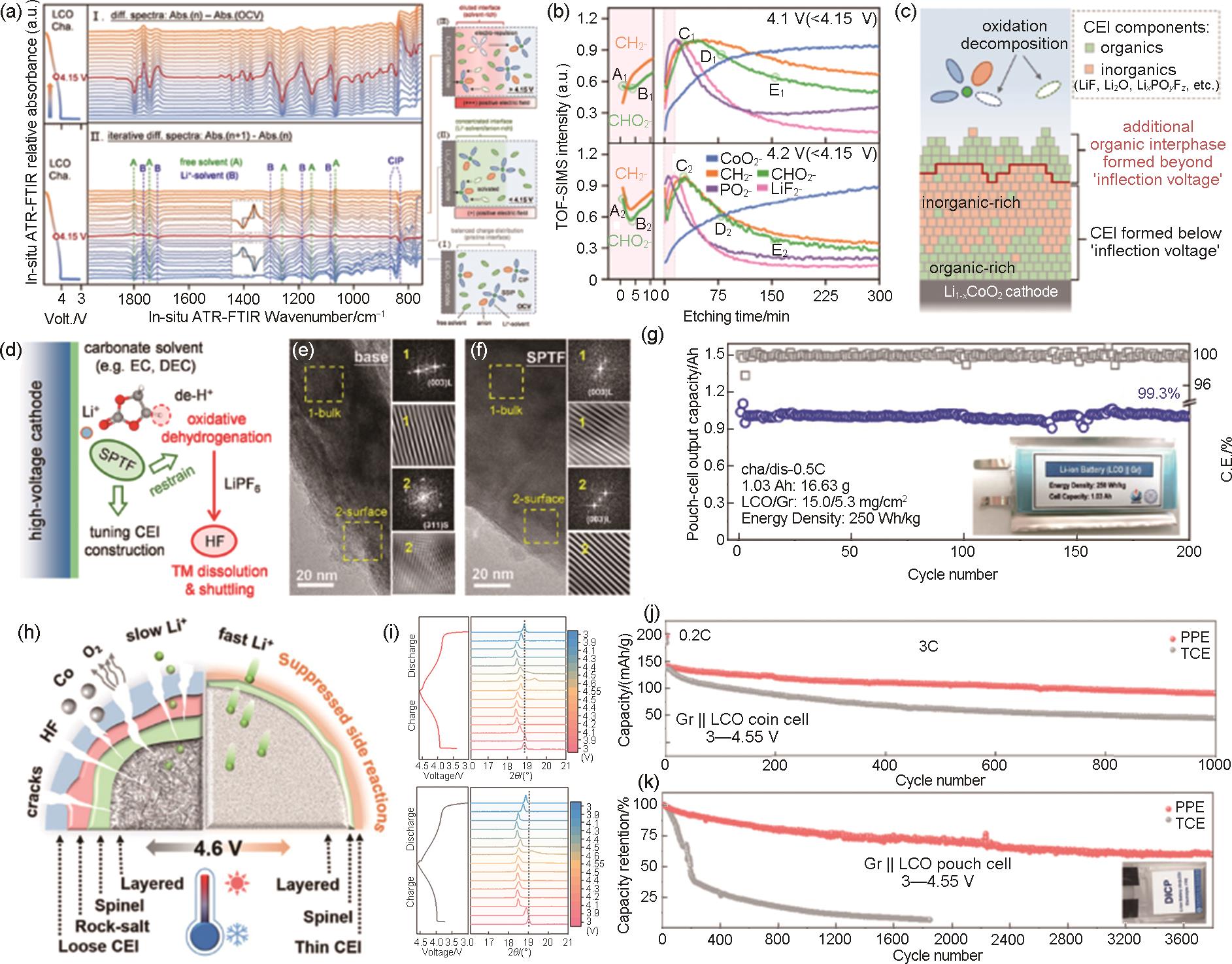
Fig. 19
(a) Representative functional groups of sulfone, sulfonate, and sulfate (left), and HOMO-LUMO energy levels of EC, SU, PS, DT, and MS (right); (b) The initial CE and (c) average CE of LCO-Li cells at different charge voltages (the charge cutoff voltages are 4.2, 4.4, and 4.6 V from left to right for every species); XPS spectra of (d) S 2p and (e) O 1s on the LCO surface at various charge voltages after 100 cycles; (f) The function mechanism of the MS additive for high-voltage LCO[165]; (g) Calculated HOMO and LUMO of different molecular; (h) The TEM images of LCO particles cycled in BE (up) and DE (down) after 50 cycles at 1C; (i) In-situ Raman spectra of the LCO in BE and DE at 0.5 mV/s upon the first cycle; (j) The selected Raman spectra at various charge/discharge states. TEM image of LCO particle after 2 cycles in (k) BE and (l) DE at 0.2 C; (m) The contents of transition metal Co ions in different electrolytes after 10, 50 and 100 cycles[166]"
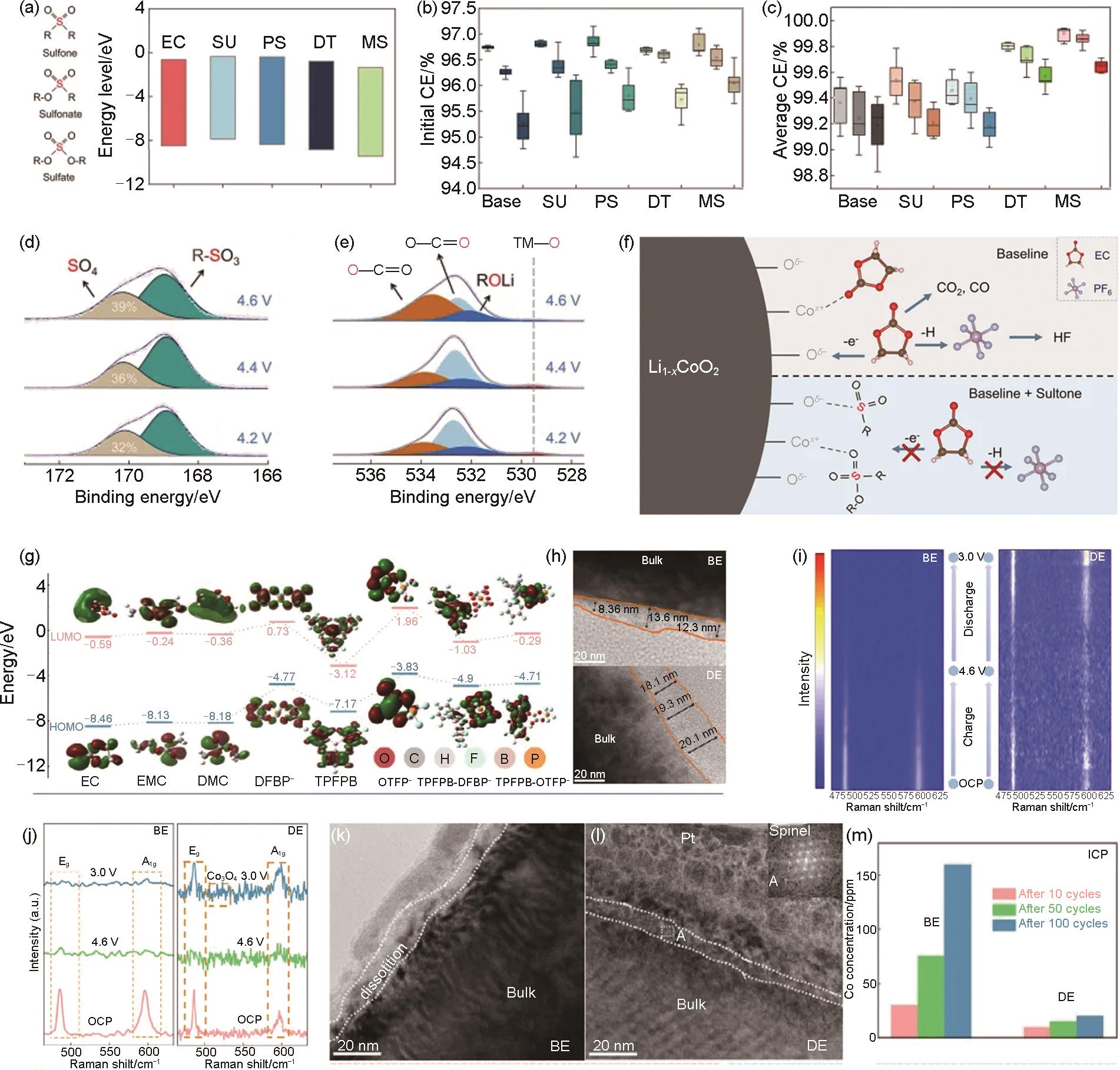
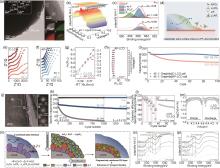
Fig. 20
(a) TEM images and EDS mapping results of AP-LCO; (b) Evolution of Al-O-P signals in IR results upon 1st charging; (c) Evolution of the F 1s XPS results during cycles under 45 ℃; (d) Schematic diagram of chemical and morphological evolution of CEI on AP-LCO. Comparison of the temperature-dependent EIS spectra of (e) C-LCO and (f) AP-LCO; (g) Eavalues of C-LCO and AP-LCO in the 102th cycle under 45 ℃; (h) RCEI values in the 2nd and 102th cycle for AP-LCO under 45 ℃; (i) Comparison of the cycle stability curves at 1C in 3—4.6 V under 45 ℃ in Graphite||LCO cells[169]; (j) HRTEM image and corresponding FFT results of Z-LCO; (k) Cyclability at 1C, (l) Rate performance, and (m) GITT tests of LCO and Z-LCO between 3—4.6 V; (n) The schematic diagram for the densifying process of CEI layer for Z-LCO; O K-edge spectra of TEY mode from sXAS measurements of (o) LCO and (p) Z-LCO during 1st and 10th cycle[170]"
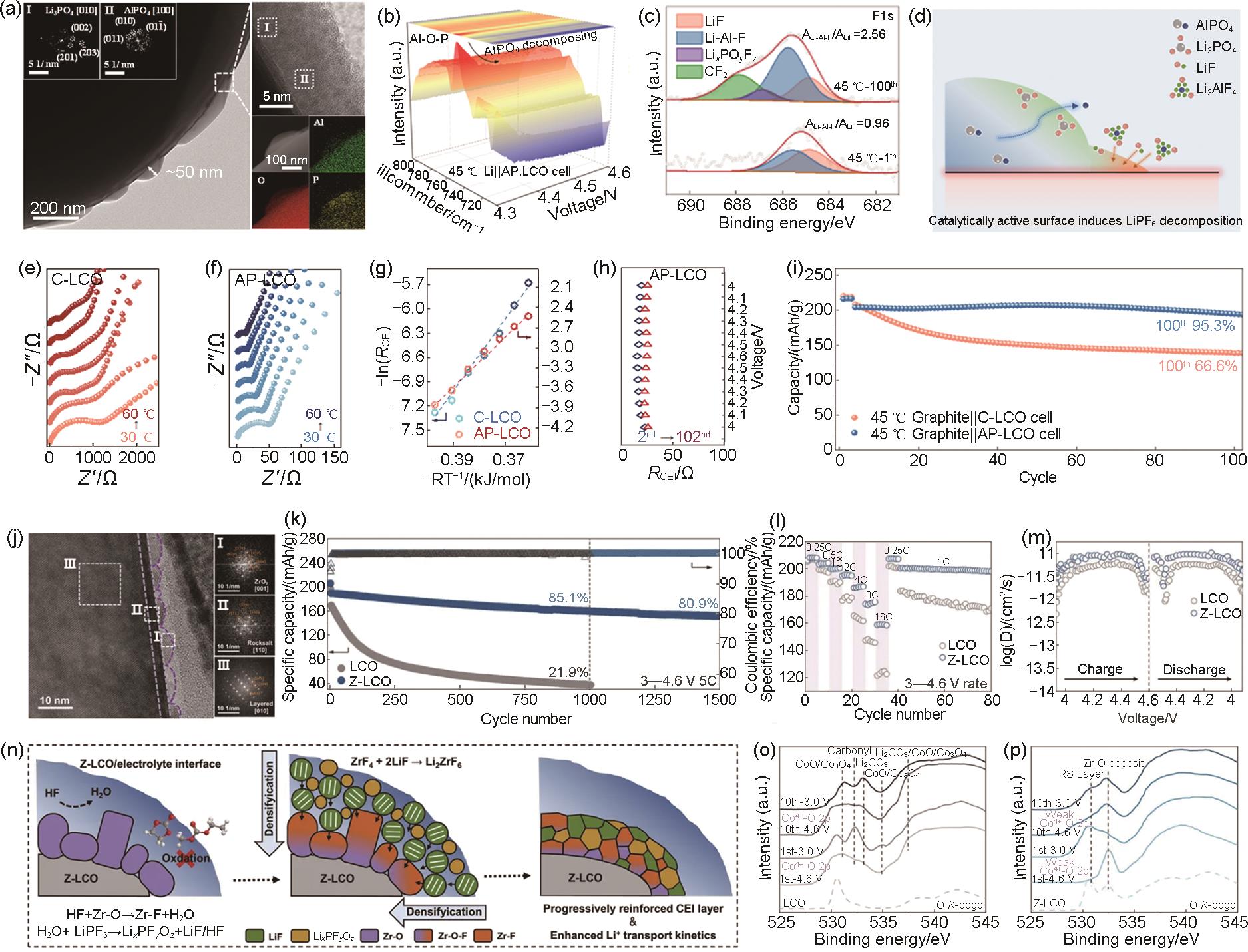

Fig. 21
In-situ XRD patterns of LCO electrodes with (a) PVDF and (b) FUC-PAM binder; (c, d) HRTEM images and fast Fourier transform (FFT) images (inset) of LCO electrodes with (c) PVDF and (d) FUC-PAM binder after 200 cycles; Co L-edge XAS patterns in TEY mode of LCO electrodes with (e) PVDF and (f) FUC-PAM binder; (g) Differential charge density of the LCO electrode with FUC-PAM binder; EIS spectra for electrodes at (h) pristine and (i) 100th cycle[173]"
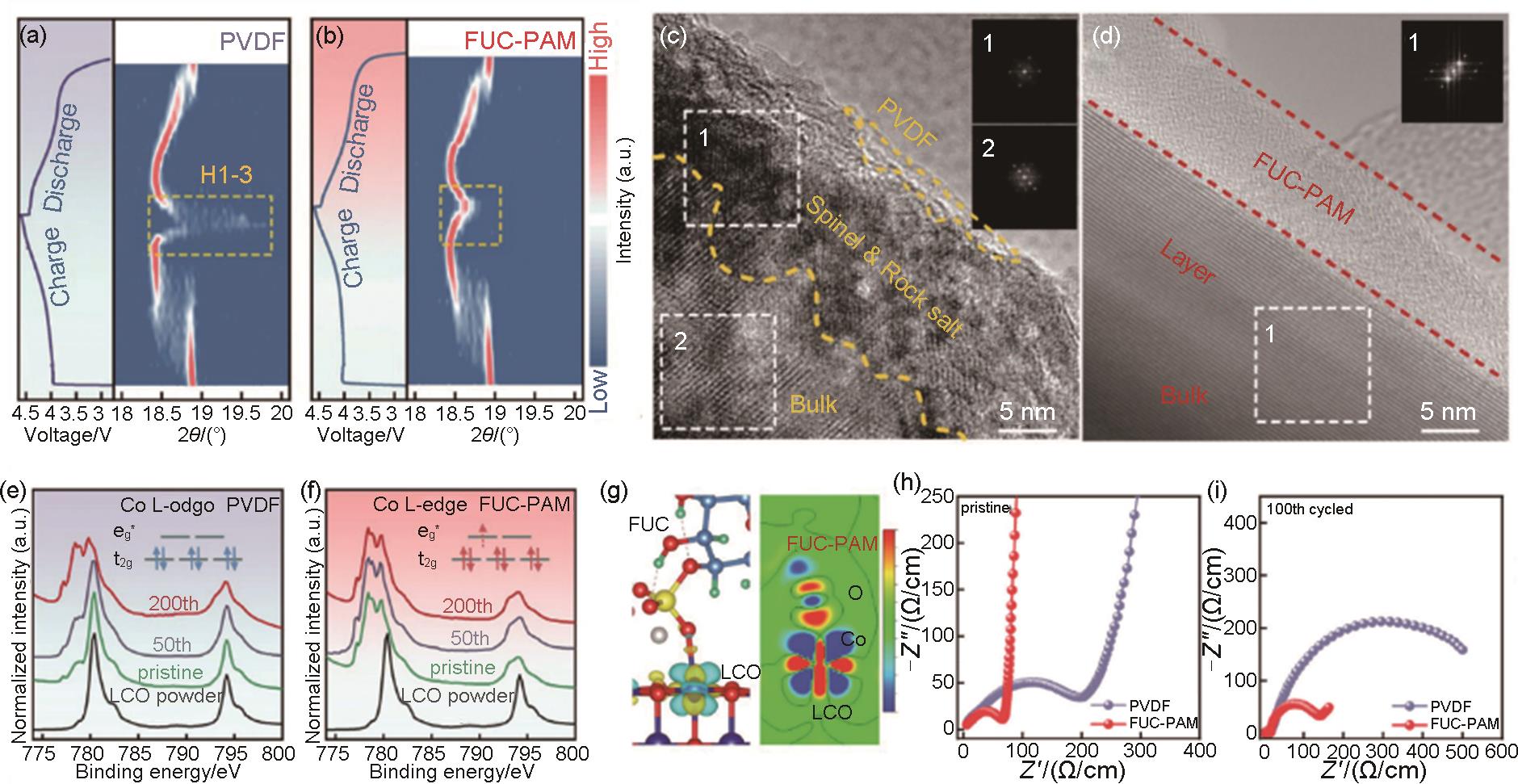
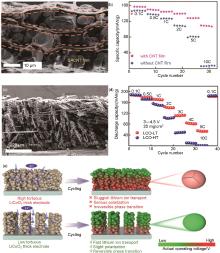
Fig. 23
(a) Cross-section SEM image of LCO, Super P, and SACNT film composite electrodes; (b) Rate performance[179]; (c) Cross-section SEM image of LCO-LT (low tortuosity); (c) Rate performance of LCO-LT and LCO-HT (high tortuosity) electrodes; (e) Illustration of the function mechanism for LCO-LT and LCO-HT electrodes[184]"
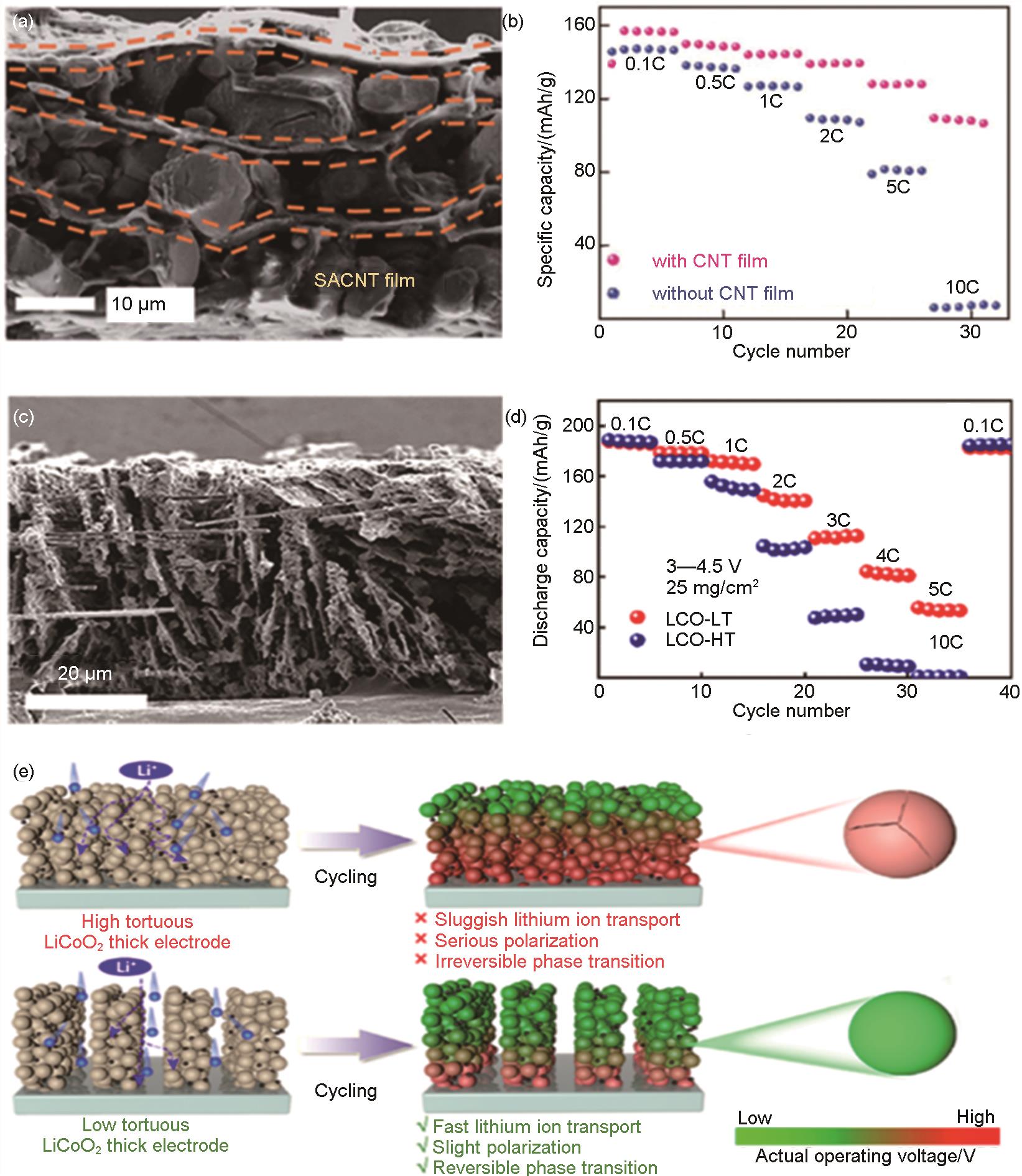
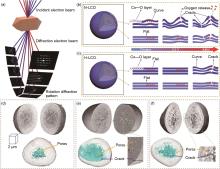
Fig. 24
Schematic illustration of (a) the data collection process of cRED, and the structural evolutions of (b) N-LCO, (c) H-LCO during charge[34] Visualizations of the internal isolated pores and interconnected crack network are highlighted (in cyan) within the reconstructed particle for (d) pristine material, and for material degraded at (e) 0.1 C and (f) 10 C[186]"
| 1 | MANTHIRAM A, GOODENOUGH J B. Layered lithium cobalt oxide cathodes[J]. Nature Energy, 2021, 6(3): 323. DOI: 10.1038/s41560-020-00764-8. |
| 2 | CHU S, MAJUMDAR A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488(7411): 294-303. DOI: 10.1038/nature11475. |
| 3 | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices[J]. Science, 2011, 334(6058): 928-935. DOI: 10.1126/science.1212741. |
| 4 | KATO Y, HORI S, SAITO T, et al. High-power all-solid-state batteries using sulfide superionic conductors[J]. Nature Energy, 2016, 1: 16030. DOI: 10.1038/nenergy.2016.30. |
| 5 | DRESSELHAUS M S, THOMAS I L. Alternative energy technologies[J]. Nature, 2001, 414(6861): 332-337. DOI: 10.1038/35104599. |
| 6 | WANG L L, CHEN B B, MA J, et al. Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density[J]. Chemical Society Reviews, 2018, 47(17): 6505-6602. DOI: 10.1039/C8CS00322J. |
| 7 | ADAM A, WANDT J, KNOBBE E, et al. Fast-charging of automotive lithium-ion cells: in situ lithium-plating detection and comparison of different cell designs[J]. Journal of the Electrochemical Society, 2020, 167(13): 130503. DOI: 10.1149/1945-7111/abb564. |
| 8 | SCROSATI B, GARCHE J. Lithium batteries: Status, prospects and future[J]. Journal of Power Sources, 2010, 195(9): 2419-2430. DOI: 10.1016/j.jpowsour.2009.11.048. |
| 9 | MEINTZ A, ZHANG J C, VIJAYAGOPAL R, et al. Enabling fast charging-vehicle considerations[J]. Journal of Power Sources, 2017, 367: 216-227. DOI: 10.1016/j.jpowsour.2017.07.093. |
| 10 | TOMASZEWSKA A, CHU Z Y, FENG X N, et al. Lithium-ion battery fast charging: A review[J]. eTransportation, 2019, 1: 100011. DOI: 10.1016/j.etran.2019.100011. |
| 11 | LI N, CHEN Z P, REN W C, et al. Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(43): 17360-17365. DOI: 10.1073/pnas.1210072109. |
| 12 | KANG B, CEDER G. Battery materials for ultrafast charging and discharging[J]. Nature, 2009, 458(7235): 190-193. DOI: 10.1038/nature07853. |
| 13 | ZHANG S S. The effect of the charging protocol on the cycle life of a Li-ion battery[J]. Journal of Power Sources, 2006, 161(2): 1385-1391. DOI: 10.1016/j.jpowsour.2006.06.040. |
| 14 | GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries[J]. Chemistry of Materials, 2010, 22(3): 587-603. DOI: 10.1021/cm901452z. |
| 15 | PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 1997, 144(4): 1188. DOI: 10.1149/1.1837571. |
| 16 | ZHENG J M, MYEONG S, CHO W, et al. Li- and Mn-rich cathode materials: Challenges to commercialization[J]. Advanced Energy Materials, 2017, 7(6): 1601284. DOI: 10.1002/aenm.201601284. |
| 17 | PARK J Y, JO M, HONG S, et al. Mechanism of degradation of capacity and charge/discharge voltages of high-Ni cathode during fast long-term cycling without voltage margin[J]. Advanced Energy Materials, 2022, 12(29): 2201151. DOI: 10.1002/aenm. 202201151. |
| 18 | WANG R, CHEN X, HUANG Z Y, et al. Twin boundary defect engineering improves lithium-ion diffusion for fast-charging spinel cathode materials[J]. Nature Communications, 2021, 12(1): 3085. DOI: 10.1038/s41467-021-23375-7. |
| 19 | MANTHIRAM A, KNIGHT J C, MYUNG S T, et al. Nickel-rich and lithium-rich layered oxide cathodes: Progress and perspectives[J]. Advanced Energy Materials, 2016, 6(1): 1501010. DOI: 10. 1002/aenm.201501010. |
| 20 | OH J, LEE S Y, KIM H, et al. Overcharge-induced phase heterogeneity and resultant twin-like layer deformation in lithium cobalt oxide cathode for lithium-ion batteries[J]. Advanced Science, 2022, 9(32): 2203639. DOI: 10.1002/advs.202203639. |
| 21 | JOHNSTON W D, HEIKES R R, SESTRICH D. The preparation, crystallography, and magnetic properties of the LixCo(1- x)O system[J]. Journal of Physics and Chemistry of Solids, 1958, 7(1): 1-13. DOI: 10.1016/0022-3697(58)90175-6. |
| 22 | DELMAS C, FOUASSIER C, HAGENMULLER P. Structural classification and properties of the layered oxides[J]. Physica B+C, 1980, 99(1): 81-85. DOI: 10.1016/0378-4363(80)90214-4. |
| 23 | LIN C, LI J Y, YIN Z W, et al. Structural understanding for high-voltage stabilization of lithium cobalt oxide[J]. Advanced Materials, 2024, 36(6): 2307404. DOI: 10.1002/adma.202307404. |
| 24 | THACKERAY M M, DAVID W I F, BRUCE P G, et al. Lithium insertion into manganese spinels[J]. Materials Research Bulletin, 1983, 18(4): 461-472. DOI: 10.1016/0025-5408(83)90138-1. |
| 25 | WANG J J, SUN X L. Olivine LiFePO4: The remaining challenges for future energy storage[J]. Energy & Environmental Science, 2015, 8(4): 1110-1138. DOI: 10.1039/C4EE04016C. |
| 26 | ENSLING D, CHERKASHININ G, SCHMID S, et al. Nonrigid band behavior of the electronic structure of LiCoO2 thin film during electrochemical Li deintercalation[J]. Chemistry of Materials, 2014, 26(13): 3948-3956. DOI: 10.1021/cm501480b. |
| 27 | AMATUCCI G G, TARASCON J M, KLEIN L C. Cobalt dissolution in LiCoO2-based non-aqueous rechargeable batteries[J]. Solid State Ionics, 1996, 83(1/2): 167-173. DOI: 10.1016/0167-2738(95)00231-6. |
| 28 | WU Z X, ZENG G F, YIN J H, et al. Unveiling the evolution of LiCoO2 beyond 4.6 V[J]. ACS Energy Letters, 2023, 8(11): 4806-4817. DOI: 10.1021/acsenergylett.3c01954. |
| 29 | VAN DER VEN A, AYDINOL M K, CEDER G, et al. First-principles investigation of phase stability in LixCoO2[J]. Physical Review B, 1998, 58(6): 2975-2987. DOI: 10.1103/physrevb. 58.2975. |
| 30 | NI L S, ZHANG S, DI A D, et al. Challenges and strategies towards single-crystalline Ni-rich layered cathodes[J]. Advanced Energy Materials, 2022, 12(31): 2201510. DOI: 10.1002/aenm. 202201510. |
| 31 | CHEN Z H, DAHN J R. Methods to obtain excellent capacity retention in LiCoO2 cycled to 4.5 V[J]. Electrochimica Acta, 2004, 49(7): 1079-1090. DOI: 10.1016/j.electacta.2003.10.019. |
| 32 | LI S, SUN Y, GAO A, et al. Sustainable LiCoO2 by collective glide of CoO6 slabs upon charge/discharge[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(20): e2120060119. DOI: 10.1073/pnas.2120060119. |
| 33 | WANG X H, REN H Y, DU Y H, et al. Tuning surface chemistry to reduce the step-like degradation of LiCoO2 at 4.6 V[J]. Nano Energy, 2024, 125: 109537. DOI: 10.1016/j.nanoen.2024.109537. |
| 34 | LI J Y, LIN C, WENG M Y, et al. Structural origin of the high-voltage instability of lithium cobalt oxide[J]. Nature Nanotechnology, 2021, 16(5): 599-605. DOI: 10.1038/s41565-021-00855-x. |
| 35 | WANG Z X, LIU L J, CHEN L Q, et al. Structural and electrochemical characterizations of surface-modified LiCoO2 cathode materials for Li-ion batteries[J]. Solid State Ionics, 2002, 148(3/4): 335-342. DOI: 10.1016/S0167-2738(02)00071-1. |
| 36 | MA X S, WANG J K, WANG Z H, et al. Engineering strategies for high-voltage LiCoO2 based high-energy Li-ion batteries[J]. Electron, 2024, 2(3): e33. DOI: 10.1002/elt2.33. |
| 37 | ZHANG J N, LI Q H, OUYANG C Y, et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V[J]. Nature Energy, 2019, 4(7): 594-603. DOI: 10.1038/s41560-019-0409-z. |
| 38 | YU Z Z, TONG Q L, ZHAO G Q, et al. Combining surface holistic Ge coating and subsurface Mg doping to enhance the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathodes[J]. ACS Applied Materials & Interfaces, 2022, 14(22): 25490-25500. DOI: 10.1021/acsami.2c04666. |
| 39 | CEDER G, CHIANG Y M, SADOWAY D R, et al. Identification of cathode materials for lithium batteries guided by first-principles calculations[J]. Nature, 1998, 392(6677): 694-696. DOI: 10.1038/33647. |
| 40 | WANG H S, RUS E, SAKURABA T, et al. CO2 and O2 evolution at high voltage cathode materials of Li-ion batteries: A differential electrochemical mass spectrometry study[J]. Analytical Chemistry, 2014, 86(13): 6197-6201. DOI: 10.1021/ac403317d. |
| 41 | TAKAMATSU D, KOYAMA Y, ORIKASA Y, et al. First In Situ observation of the LiCoO2 electrode/electrolyte interface by total-reflection X-ray absorption spectroscopy[J]. Angewandte Chemie International Edition, 2012, 51(46): 11597-11601. DOI: 10.1002/anie.201203910. |
| 42 | TAKAMATSU D, NAKATSUTSUMI T, MORI S, et al. Nanoscale observation of the electronic and local structures of LiCoO2 thin film electrode by depth-resolved X-ray absorption spectroscopy[J]. The Journal of Physical Chemistry Letters, 2011, 2(20): 2511-2514. DOI: 10.1021/jz2011226. |
| 43 | TEBBE J L, HOLDER A M, MUSGRAVE C B. Mechanisms of LiCoO2 cathode degradation by reaction with HF and protection by thin oxide coatings[J]. ACS Applied Materials & Interfaces, 2015, 7(43): 24265-24278. DOI: 10.1021/acsami.5b07887. |
| 44 | NAKAYAMA K, KOBAYASHI S, ISHIKAWA R, et al. Atomic-scale and nanoscale structural changes in LiCoO2 due to irreversible delithiation accompanied by oxygen release[J]. Chemistry of Materials, 2024: acs.chemmater.4c02084. DOI: 10.1021/acs.chemmater.4c02084. |
| 45 | SONG Y Z, WANG L, SHENG L, et al. The significance of mitigating crosstalk in lithium-ion batteries: A review[J]. Energy & Environmental Science, 2023, 16(5): 1943-1963. DOI: 10.1039/D3EE00441D. |
| 46 | ZHANG S S. Understanding of performance degradation of LiNi0.80Co0.10Mn0.10O2 cathode material operating at high potentials[J]. Journal of Energy Chemistry, 2020, 41: 135-141. DOI: 10.1016/j.jechem.2019.05.013. |
| 47 | XIA H, LU L, MENG Y S, et al. Phase transitions and high-voltage electrochemical behavior of LiCoO2 thin films grown by pulsed laser deposition[J]. Journal of the Electrochemical Society, 2007, 154(4): A337. DOI: 10.1149/1.2509021. |
| 48 | SEONG W M, YOON K, LEE M H, et al. Unveiling the intrinsic cycle reversibility of a LiCoO2 electrode at 4.8-V cutoff voltage through subtractive surface modification for lithium-ion batteries[J]. Nano Lett, 2019, 19(1): 29-37. DOI: 10.1021/acs.nanolett.8b02902. |
| 49 | JIANG Y Y, QIN C D, YAN P F, et al. Origins of capacity and voltage fading of LiCoO2 upon high voltage cycling[J]. Journal of Materials Chemistry A, 2019, 7(36): 20824-20831. DOI: 10.1039/C9TA06579B. |
| 50 | YANG X G, ZHANG G S, GE S H, et al. Fast charging of lithium-ion batteries at all temperatures[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(28): 7266-7271. DOI: 10.1073/pnas.1807115115. |
| 51 | XIA H R, ZHANG W, CAO S K, et al. A figure of merit for fast-charging Li-ion battery materials[J]. ACS Nano, 2022, 16(6): 8525-8530. DOI: 10.1021/acsnano.2c03922. |
| 52 | ZHANG Y X, KIM J C, SONG H W, et al. Recent achievements toward the development of Ni-based layered oxide cathodes for fast-charging Li-ion batteries[J]. Nanoscale, 2023, 15(9): 4195-4218. DOI: 10.1039/d2nr05701h. |
| 53 | XU Z R, JIANG Z S, KUAI C G, et al. Charge distribution guided by grain crystallographic orientations in polycrystalline battery materials[J]. Nature Communications, 2020, 11(1): 83. DOI: 10.1038/s41467-019-13884-x. |
| 54 | BESLI M M, SUBBARAMAN A, POUR SAFAEI F R, et al. A study of model-based protective fast-charging and associated degradation in commercial smartphone cells: Insights on cathode degradation as a result of lithium depositions on the anode[J]. Advanced Energy Materials, 2021, 11(12): 2003019. DOI: 10.1002/aenm.202003019. |
| 55 | JIANG Z S, LI J Z, YANG Y, et al. Machine-learning-revealed statistics of the particle-carbon/binder detachment in lithium-ion battery cathodes[J]. Nature Communications, 2020, 11(1): 2310. DOI: 10.1038/s41467-020-16233-5. |
| 56 | TANIM T R, YANG Z Z, COLCLASURE A M, et al. Extended cycle life implications of fast charging for lithium-ion battery cathode[J]. Energy Storage Materials, 2021, 41: 656-666. DOI: 10.1016/j.ensm.2021.07.001. |
| 57 | 杲齐新, 赵景腾, 李国兴. 锂离子电池快速充电研究进展[J]. 储能科学与技术, 2023, 12(7): 2166-2184. DOI: 10.19799/j.cnki.2095-4239.2023.0287. |
| GAO Q X, ZHAO J T, LI G X. Research progress on fast-charging lithium-ion batteries[J]. Energy Storage Science and Technology, 2023, 12(7): 2166-2184. DOI: 10.19799/j.cnki.2095-4239.2023.0287. | |
| 58 | YANG Y, XU R, ZHANG K, et al. Quantification of heterogeneous degradation in Li-ion batteries[J]. Advanced Energy Materials, 2019, 9(25): 1900674. DOI: 10.1002/aenm.201900674. |
| 59 | PARK J, ZHAO H B, KANG S D, et al. Fictitious phase separation in Li layered oxides driven by electro-autocatalysis[J]. Nature Materials, 2021, 20(7): 991-999. DOI: 10.1038/s41563-021-00936-1. |
| 60 | LIU W G, ZHENG J Q, ZHANG Z, et al. The capacity decay mechanism of the 100% SOC LiCoO2/graphite battery after high-temperature storage[J]. Journal of Power Sources, 2023, 580: 233330. DOI: 10.1016/j.jpowsour.2023.233330. |
| 61 | SUN Z Y, LI F K, DING J Y, et al. High-voltage and high-temperature LiCoO2 operation via the electrolyte additive of electron-defect boron compounds[J]. ACS Energy Letters, 2023, 8(6): 2478-2487. DOI: 10.1021/acsenergylett.3c00324. |
| 62 | MENG T, YANG S S, PENG Y T, et al. Deciphering interphase instability of lithium metal batteries with localized high-concentration electrolytes at elevated temperatures[J]. Energy Storage Materials, 2024, 71: 103598. DOI: 10.1016/j.ensm.2024.103598. |
| 63 | SHEN Y B, WANG L C, JIANG J Z, et al. Stabilization of high-voltage layered oxide cathode by multi-electron rare earth oxide[J]. Chemical Engineering Journal, 2023, 454: 140249. DOI: 10.1016/j.cej.2022.140249. |
| 64 | ZHANG Z J, ZHANG H Z, WU Y P, et al. Advances in doping strategies for sodium transition metal oxides cathodes: A review[J]. Frontiers in Energy, 2024, 18(2): 141-159. DOI: 10.1007/s11708-024-0918-8. |
| 65 | HUANG Y Y, ZHU Y C, FU H Y, et al. Mg-pillared LiCoO2: Towards stable cycling at 4.6 V[J]. Angewandte Chemie International Edition, 2021, 60(9): 4682-4688. DOI: 10.1002/anie.202014226. |
| 66 | YOON M, DONG Y H, YOO Y, et al. Unveiling nickel chemistry in stabilizing high-voltage cobalt-rich cathodes for lithium-ion batteries[J]. Advanced Functional Materials, 2020, 30(6): 1907903. DOI: 10.1002/adfm.201907903. |
| 67 | KIM J, KANG H, GO N, et al. Egg-shell structured LiCoO2 by Cu2+ substitution to Li+ sites via facile stirring in an aqueous copper(ii) nitrate solution[J]. Journal of Materials Chemistry A, 2017, 5(47): 24892-24900. DOI: 10.1039/C7TA07232E. |
| 68 | ZHANG J N, LI Q H, LI Q, et al. Improved electrochemical performances of high voltage LiCoO2 with tungsten doping[J]. Chinese Physics B, 2018, 27(8): 088202. DOI: 10.1088/1674-1056/27/8/088202. |
| 69 | TAN X H, ZHAO T Q, SONG L T, et al. Simultaneous near-surface trace doping and surface modifications by gas-solid reactions during one-pot synthesis enable stable high-voltage performance of LiCoO2[J]. Advanced Energy Materials, 2022, 12(30): 2200008. DOI: 10.1002/aenm.202200008. |
| 70 | ER-RAMI F E, DUFFIET M, HINKLE S, et al. Understanding the role of Al doping of LiCoO2 on the mechanisms upon cycling up to high voltages (≥4.6 V vs Li+/Li)[J]. Chemistry of Materials, 2022, 34(10): 4384-4393. DOI: 10.1021/acs.chemmater.1c04338. |
| 71 | KONG W J, WONG D, AN K, et al. Stabilizing the anionic redox in 4.6 V LiCoO2 cathode through adjusting oxygen magnetic moment[J]. Advanced Functional Materials, 2022, 32(31): 2202679. DOI: 10.1002/adfm.202202679. |
| 72 | KIM S H, KIM C S. Improving the rate performance of LiCoO2 by Zr doping[J]. Journal of Electroceramics, 2009, 23(2): 254-257. DOI: 10.1007/s10832-008-9414-5. |
| 73 | ZHU Z, WANG H, LI Y, et al. A surface Se-substituted LiCo[O2- δSeδ] cathode with ultrastable high-voltage cycling in pouch full-cells[J]. Advanced Materials, 2020, 32(50): 2005182. DOI: 10.1002/adma.202005182. |
| 74 | LIU Q, SU X, LEI D, et al. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping[J]. Nature Energy, 2018, 3(11): 936-943. DOI: 10.1038/s41560-018-0180-6. |
| 75 | HUANG W Y, ZHAO Q, ZHANG M J, et al. Surface design with cation and anion dual gradient stabilizes high-voltage LiCoO2[J]. Advanced Energy Materials, 2022, 12(20): 2200813. DOI: 10.1002/aenm.202200813. |
| 76 | KONG W J, ZHANG J C, WONG D, et al. Tailoring Co3d and O2p band centers to inhibit oxygen escape for stable 4.6 V LiCoO2 cathodes[J]. Angewandte Chemie International Edition, 2021, 60(52): 27102-27112. DOI: 10.1002/anie.202112508. |
| 77 | LIU A, LI J, SHUNMUGASUNDARAM R, et al. Synthesis of Mg and Mn doped LiCoO2 and effects on high voltage cycling[J]. Journal of the Electrochemical Society, 2017, 164(7): A1655-A1664. DOI: 10.1149/2.1381707jes. |
| 78 | SONG S C, LI Y W, YANG K, et al. Interplay between multiple doping elements in high-voltage LiCoO2[J]. Journal of Materials Chemistry A, 2021, 9(9): 5702-5710. DOI: 10.1039/d0ta09931g. |
| 79 | CHEN S X, WANG C W, ZHOU Y, et al. Co/Li-dual-site doping towards LiCoO2 as a high-voltage, fast-charging, and long-cycling cathode material[J]. Journal of Materials Chemistry A, 2022, 10(10): 5295-5304. DOI: 10.1039/D1TA10612K. |
| 80 | LI X Q, ZHOU L M, WANG H, et al. Dopants modulate crystal growth in molten salts enabled by surface energy tuning[J]. Journal of Materials Chemistry A, 2021, 9(35): 19675-19680. DOI: 10.1039/D1TA02351A. |
| 81 | YAO Z Q, FU T J, PAN T, et al. Modulating the spin state to stabilize the surface and bulk structure for durable 4.6 V LiCoO2 cathodes[J]. Advanced Functional Materials, 2024, 34(48): 2408152. DOI: 10.1002/adfm.202408152. |
| 82 | WU Q, ZHANG B, LU Y Y. Progress and perspective of high-voltage lithium cobalt oxide in lithium-ion batteries[J]. Journal of Energy Chemistry, 2022, 74: 283-308. DOI: 10.1016/j.jechem. 2022.07.007. |
| 83 | JUNG H G, GOPAL N V, PRAKASH J, et al. Improved electrochemical performances of LiM0.05Co0.95O1.95F0.05 (M=Mg, Al, Zr) at high voltage[J]. Electrochimica Acta, 2012, 68: 153-157. DOI: 10.1016/j.electacta.2012.02.057. |
| 84 | HU B, LOU X B, LI C, et al. Reversible phase transition enabled by binary Ba and Ti-based surface modification for high voltage LiCoO2 cathode[J]. Journal of Power Sources, 2019, 438: 226954. DOI: 10.1016/j.jpowsour.2019.226954. |
| 85 | CHENG J H, PAN C J, NITHYA C, et al. Effect of Mg doping on the local structure of LiMgyCo1- yO2 cathode material investigated by X-ray absorption spectroscopy[J]. Journal of Power Sources, 2014, 252: 292-297. DOI: 10.1016/j.jpowsour.2013.10.130. |
| 86 | ZHANG W, ZHANG X Y, CHENG F Y, et al. Enabling stable 4.6 V LiCoO2 cathode through oxygen charge regulation strategy[J]. Journal of Energy Chemistry, 2023, 76: 557-565. DOI: 10.1016/j.jechem.2022.09.034. |
| 87 | YOON W S, KIM K B, KIM M G, et al. Oxygen contribution on Li-ion intercalation-deintercalation in LiAlyCo1- yO2 investigated by O K-edge and co L-edge X-ray absorption spectroscopy[J]. Journal of the Electrochemical Society, 2002, 149(10): A1305. DOI: 10.1149/1.1503074. |
| 88 | ZHU Z, KUSHIMA A, YIN Z Y, et al. Anion-redox nanolithia cathodes for Li-ion batteries[J]. Nature Energy, 2016, 1: 16111. DOI: 10.1038/nenergy.2016.111. |
| 89 | QIAO Y, JIANG K Z, DENG H, et al. A high-energy-density and long-life lithium-ion battery via reversible oxide-peroxide conversion[J]. Nature Catalysis, 2019, 2(11): 1035-1044. DOI: 10.1038/s41929-019-0362-z. |
| 90 | YOON W S, KIM K B, KIM M G, et al. Oxygen contribution on Li-ion intercalation-deintercalation in LiCoO2 investigated by O K-edge and co L-edge X-ray absorption spectroscopy[J]. The Journal of Physical Chemistry B, 2002, 106(10): 2526-2532. DOI: 10.1021/jp013735e. |
| 91 | KANNAN A M, RABENBERG L, MANTHIRAM A. High capacity surface-modified LiCoO2 cathodes for lithium-ion batteries[J]. Electrochemical and Solid-State Letters, 2003, 6(1): A16-A18. DOI: 10.1149/1.1526782. |
| 92 | LEE E, PERSSON K A. Structural and chemical evolution of the layered Li-excess LixMnO3 as a function of Li content from first-principles calculations[J]. Advanced Energy Materials, 2014, 4(15): 1400498. DOI: 10.1002/aenm.201400498. |
| 93 | YAN P F, ZHENG J M, TANG Z K, et al. Injection of oxygen vacancies in the bulk lattice of layered cathodes[J]. Nature Nanotechnology, 2019, 14(6): 602-608. DOI: 10.1038/s41565-019-0428-8. |
| 94 | ZHANG D H, WANG J Q, LIN Y G, et al. Present situation and future prospect of renewable energy in China[J]. Renewable and Sustainable Energy Reviews, 2017, 76: 865-871. DOI: 10.1016/j.rser.2017.03.023. |
| 95 | SI Z, SHI B Z, HUANG J, et al. Titanium and fluorine synergetic modification improves the electrochemical performance of Li(Ni0.8Co0.1Mn0.1)O2[J]. Journal of Materials Chemistry A, 2021, 9(14): 9354-9363. DOI: 10.1039/D1TA00124H. |
| 96 | YANG S Q, WANG P B, WEI H X, et al. Li4V2Mn(PO4)4-stablized Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials for lithium ion batteries[J]. Nano Energy, 2019, 63: 103889. DOI: 10.1016/j.nanoen.2019.103889. |
| 97 | SUN Z H, XU L Q, DONG C Q, et al. A facile gaseous sulfur treatment strategy for Li-rich and Ni-rich cathode materials with high cycling and rate performance[J]. Nano Energy, 2019, 63: 103887. DOI: 10.1016/j.nanoen.2019.103887. |
| 98 | ZHU Z, WANG H, LI Y, et al. A surface Se-substituted LiCo[O2- δSeδ] cathode with ultrastable high-voltage cycling in pouch full-cells[J]. Advanced Materials,2020. |
| 99 | HUANG Y Y, ZHU Y C, FU H Y, et al. Mg-pillared LiCoO2: Towards stable cycling at 4.6 V[J]. Angewandte Chemie International Edition, 2021, 60(9): 4682-4688. DOI: 10.1002/anie.202014226. |
| 100 | YAO Y T, XUE Z Y, LI C Y, et al. Progress and perspective of doping strategies for lithium cobalt oxide materials in lithium-ion batteries[J]. Energy Storage Materials, 2024, 71: 103666. DOI: 10.1016/j.ensm.2024.103666. |
| 101 | CHO W, MYEONG S, KIM N, et al. Critical role of cations in lithium sites on extended electrochemical reversibility of co-rich layered oxide[J]. Advanced Materials, 2017, 29(21): 1605578. DOI: 10.1002/adma.201605578. |
| 102 | GOODENOUGH J B, PARK K S. The Li-ion rechargeable battery: A perspective[J]. Journal of the American Chemical Society, 2013, 135(4): 1167-1176. DOI: 10.1021/ja3091438. |
| 103 | WANG M J, YU F D, SUN G, et al. Co-regulating the surface and bulk structure of Li-rich layered oxides by a phosphor doping strategy for high-energy Li-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(14): 8302-8314. DOI: 10.1039/C9TA00783K. |
| 104 | TAN X H, ZHAO T Q, SONG L T, et al. Simultaneous near-surface trace doping and surface modifications by gas-solid reactions during one-pot synthesis enable stable high-voltage performance of LiCoO2[J]. Advanced Energy Materials, 2022, 12(30): 2200008. DOI: 10.1002/aenm.202200008. |
| 105 | ZHENG W, LIANG G M, GUO H, et al. Enhancing the reaction kinetics and structural stability of high-voltage LiCoO2 via polyanionic species anchoring[J]. Energy & Environmental Science, 2024, 17(12): 4147-4156. DOI: 10.1039/D4EE00726C. |
| 106 | MIN S H, JO M R, CHOI S Y, et al. A layer-structured electrode material reformed by a PO4 -O2 hybrid framework toward enhanced lithium storage and stability[J]. Advanced Energy Materials, 2016, 6(7): 1501717. DOI: 10.1002/aenm.201501717. |
| 107 | WAN J J, ZHU J P, XIANG Y X, et al. Revealing the correlation between structure evolution and electrochemical performance of high-voltage lithium cobalt oxide[J]. Journal of Energy Chemistry, 2021, 54: 786-794. DOI: 10.1016/j.jechem.2020.06.027. |
| 108 | HONG Y S, HUANG X J, WEI C X, et al. Hierarchical defect engineering for LiCoO2 through low-solubility trace element doping[J]. Chem, 2020, 6(10): 2759-2769. DOI: 10.1016/j.chempr.2020.07.017. |
| 109 | WANG L L, MA J, WANG C, et al. A novel bifunctional self-stabilized strategy enabling 4.6 V LiCoO2 with excellent long-term cyclability and high-rate capability[J]. Advanced Science, 2019, 6(12): 1900355. DOI: 10.1002/advs.201900355. |
| 110 | ZHANG Z J, WANG J, SUN X Y, et al. Improved stability of single-crystal LiCoO2 cathodes at 4.8 V through solvation structure regulation[J]. Advanced Functional Materials, 2024, 34(48): 2411409. DOI: 10.1002/adfm.202411409. |
| 111 | LIU T C, YU L, LIU J J, et al. Understanding Co roles towards developing co-free Ni-rich cathodes for rechargeable batteries[J]. Nature Energy, 2021, 6(3): 277-286. DOI: 10.1038/s41560-021-00776-y. |
| 112 | CHAE B G, PARK S Y, SONG J H, et al. Evolution and expansion of Li concentration gradient during charge-discharge cycling[J]. Nature Communications, 2021, 12(1): 3814. DOI: 10.1038/s41467-021-24120-w. |
| 113 | BI Y J, TAO J H, WU Y Q, et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode[J]. Science, 2020, 370(6522): 1313-1317. DOI: 10.1126/science.abc3167. |
| 114 | ZHU Z, YU D W, SHI Z, et al. Gradient-morph LiCoO2 single crystals with stabilized energy density above 3400 Wh·L-1[J]. Energy & Environmental Science, 2020, 13(6): 1865-1878. DOI: 10.1039/D0EE00231C. |
| 115 | KANG K, CEDER G. Factors that affect Li mobility in layered lithium transition metal oxides[J]. Physical Review B, 2006, 74(9): 094105. DOI: 10.1103/physrevb.74.094105. |
| 116 | LU W, ZHANG J S, XU J J, et al. In situ visualized cathode electrolyte interphase on LiCoO2 in high voltage cycling[J]. ACS Applied Materials & Interfaces, 2017, 9(22): 19313-19318. DOI: 10.1021/acsami.7b03024. |
| 117 | ZHOU A J, LIU Q, WANG Y, et al. Al2O3 surface coating on LiCoO2 through a facile and scalable wet-chemical method towards high-energy cathode materials withstanding high cutoff voltages[J]. Journal of Materials Chemistry A, 2017, 5(46): 24361-24370. DOI: 10.1039/C7TA07312G. |
| 118 | WU R, CAO T C, LIU H, et al. Ultralong lifespan for high-voltage LiCoO2 enabled by in situ reconstruction of an atomic layer deposition coating layer[J]. ACS Applied Materials & Interfaces, 2022, 14(22): 25524-25533. DOI: 10.1021/acsami. 2c05129. |
| 119 | YASUHARA S, YASUI S, TERANISHI T, et al. Enhancement of ultrahigh rate chargeability by interfacial nanodot BaTiO3 treatment on LiCoO2 cathode thin film batteries[J]. Nano Letters, 2019, 19(3): 1688-1694. DOI: 10.1021/acs.nanolett. 8b04690. |
| 120 | CHENG T, MA Z T, QIAN R C, et al. Achieving stable cycling of LiCoO2 at 4.6 V by multilayer surface modification[J]. Advanced Functional Materials, 2021, 31(2): 2001974. DOI: 10.1002/adfm.202001974. |
| 121 | ORIKASA Y, TAKAMATSU D, YAMAMOTO K, et al. Origin of surface coating effect for MgO on LiCoO2 to improve the interfacial reaction between electrode and electrolyte[J]. Advanced Materials Interfaces, 2014, 1(9): 1400195. DOI: 10.1002/admi.201400195. |
| 122 | SUN Y K, YOON C S, MYUNG S T, et al. Role of AlF3 coating on LiCoO2 particles during cycling to cutoff voltage above 4.5 V[J]. Journal of the Electrochemical Society, 2009, 156(12): A1005. DOI: 10.1149/1.3236501. |
| 123 | GU R, MA Z T, CHENG T, et al. Improved electrochemical performances of LiCoO2 at elevated voltage and temperature with an in situ formed spinel coating layer[J]. ACS Applied Materials & Interfaces, 2018, 10(37): 31271-31279. DOI: 10.1021/acsami.8b08264. |
| 124 | TAN X H, MAO D D, ZHAO T Q, et al. Long-term highly stable high-voltage LiCoO2 synthesized via a solid sulfur-assisted one-pot approach[J]. Small, 2022, 18(26): 2202143. DOI: 10.1002/smll.202202143. |
| 125 | PANG P P, WANG Z, DENG Y M, et al. Delayed phase transition and improved cycling/thermal stability by spinel LiNi0.5Mn1.5O4 modification for LiCoO2 cathode at high voltages[J]. ACS Applied Materials & Interfaces, 2020, 12(24): 27339-27349. DOI: 10.1021/acsami.0c02459. |
| 126 | QIAN J W, LIU L, YANG J X, et al. Electrochemical surface passivation of LiCoO2 particles at ultrahigh voltage and its applications in lithium-based batteries[J]. Nature Communications, 2018, 9(1): 4918. DOI: 10.1038/s41467-018-07296-6. |
| 127 | FAN T J, KAI W, HARIKA V K, et al. Operating highly stable LiCoO2 cathodes up to 4.6 V by using an effective integration of surface engineering and electrolyte solutions selection[J]. Advanced Functional Materials, 2022, 32(33): 2204972. DOI: 10.1002/adfm.202204972. |
| 128 | XIE J, ZHAO J, LIU Y Y, et al. Engineering the surface of LiCoO2 electrodes using atomic layer deposition for stable high-voltage lithium ion batteries[J]. Nano Research, 2017, 10(11): 3754-3764. DOI: 10.1007/s12274-017-1588-1. |
| 129 | QU S S, WU W B, WU Y F, et al. Sputtering coating of lithium fluoride film on lithium cobalt oxide electrodes for reducing the polarization of lithium-ion batteries[J]. Nanomaterials, 2021, 11(12): 3393. DOI: 10.3390/nano11123393. |
| 130 | WANG P F, MENG Y, WANG Y J, et al. Oxygen framework reconstruction by LiAlH4 treatment enabling stable cycling of high-voltage LiCoO2[J]. Energy Storage Materials, 2022, 44: 487-496. DOI: 10.1016/j.ensm.2021.10.041. |
| 131 | YANG X R, WANG C W, YAN P F, et al. Pushing lithium cobalt oxides to 4.7 V by lattice-matched interfacial engineering[J]. Advanced Energy Materials, 2022, 12(23): 2200197. DOI: 10.1002/aenm.202200197. |
| 132 | ZHANG X L, PENG B, ZHAO L P, et al. NASICON-structured LiZr2(PO4)3 surface modification improves ionic conductivity and structural stability of LiCoO2 for a stable 4.6 V cathode[J]. ACS Applied Materials & Interfaces, 2022, 14(14): 16204-16213. DOI: 10.1021/acsami.2c00533. |
| 133 | WANG Y, ZHANG Q H, XUE Z C, et al. An in situ formed surface coating layer enabling LiCoO2 with stable 4.6 V high-voltage cycle performances[J]. Advanced Energy Materials, 2020, 10(28): 2001413. DOI: 10.1002/aenm.202001413. |
| 134 | SHARIFI-ASL S, SOTO F A, FOROOZAN T, et al. Anti-oxygen leaking LiCoO2[J]. Advanced Functional Materials, 2019, 29(23): 1901110. DOI: 10.1002/adfm.201901110. |
| 135 | WANG X, WU Q, LI S Y, et al. Lithium-aluminum-phosphate coating enables stable 4.6 V cycling performance of LiCoO2 at room temperature and beyond[J]. Energy Storage Materials, 2021, 37: 67-76. DOI: 10.1016/j.ensm.2021.01.031. |
| 136 | CHENG T, CHENG Q, HE Y, et al. A hybrid ionic and electronic conductive coating layer for enhanced electrochemical performance of 4.6 V LiCoO2[J]. ACS Applied Materials & Interfaces, 2021, 13(36): 42917-42926. DOI: 10.1021/acsami. 1c12882. |
| 137 | MAO S L, SHEN Z Y, ZHANG W D, et al. Outside-In nanostructure fabricated on LiCoO2 surface for high-voltage lithium-ion batteries[J]. Advanced Science, 2022, 9(11): 2104841. DOI: 10.1002/advs.202104841. |
| 138 | LUCERO M, HOLSTUN T M, YAO Y D, et al. Dual-shell silicate and alumina coating for long lasting and high capacity lithium ion batteries[J]. Journal of Energy Chemistry, 2022, 68: 314-323. DOI: 10.1016/j.jechem.2021.11.014. |
| 139 | YAO J, LI Y Y, XIONG T T, et al. Scalable precise nanofilm coating and gradient Al doping enable stable battery cycling of LiCoO2 at 4.7 V[J]. Angewandte Chemie International Edition, 2024, 63(32): e202407898. DOI: 10.1002/anie.202407898. |
| 140 | BI Z H, YI Z L, ZHANG L Z, et al. Ultrathin dense LiF coverage coupled with a near-surface gradient fluorination lattice enables fast-charging long-life 4.6 V LiCoO2[J]. Energy & Environmental Science, 2024, 17(8): 2765-2775. DOI: 10.1039/D3EE03464J. |
| 141 | ZHANG W J, QIU C F, LIN Z, et al. Modification of Li3PO4 layer effectively boosting lithium storage and thermal safety performance for LiCoO2 batteries[J]. Journal of Energy Chemistry, 2024, 99: 615-626. DOI: 10.1016/j.jechem.2024.08.010. |
| 142 | WANG J X, LIU Z M, YAN G C, et al. Improving the electrochemical performance of lithium vanadium fluorophosphate cathode material: Focus on interfacial stability[J]. Journal of Power Sources, 2016, 329: 553-557. DOI: 10.1016/j.jpowsour. 2016.08.131. |
| 143 | WU K, RAN P L, WANG B T, et al. Diffusion-optimized long lifespan 4.6 V LiCoO2: Homogenizing cycled bulk-to-surface Li concentration with reduced structure stress[J]. Advanced Science, 2024, 11(14): 2308258. DOI: 10.1002/advs.202308258. |
| 144 | DONG W J, YE B, CAI M Z, et al. Superwettable high-voltage LiCoO2 for low-temperature lithium ion batteries[J]. ACS Energy Letters, 2023, 8(2): 881-888. DOI: 10.1021/acsenergylett.2c02434. |
| 145 | ZHU R N, ZHANG S J, GUO Q X, et al. More than just a protection layer: Inducing chemical interaction between Li3BO3 and LiNi0·5Mn1·5O4 to achieve stable high-rate cycling cathode materials[J]. Electrochimica Acta, 2020, 342: 136074. DOI: 10.1016/j.electacta.2020.136074. |
| 146 | DENG Y L, MOU J R, WU H L, et al. A superior Li2SiO3-composited LiNi0.5Mn1.5O4 cathode for high-voltage and high-performance lithium-ion batteries[J]. Electrochimica Acta, 2017, 235: 19-31. DOI: 10.1016/j.electacta.2017.03.066. |
| 147 | LV D D, WANG L, HU P F, et al. Li2O-B2O3-Li2SO4 modified LiNi1/3Co1/3Mn1/3O2 cathode material for enhanced electrochemical performance[J]. Electrochimica Acta, 2017, 247: 803-811. DOI: 10.1016/j.electacta.2017.07.068. |
| 148 | JO M R, KIM Y I, KIM Y, et al. Lithium-ion transport through a tailored disordered phase on the LiNi0.5Mn1.5O4 surface for high-power cathode materials[J]. ChemSusChem, 2014, 7(8): 2248-2254. DOI: 10.1002/cssc.201402109. |
| 149 | JO M R, NAM K M, LEE Y, et al. Phosphidation of Li4Ti5O12 nanoparticles and their electrochemical and biocompatible superiority for lithium rechargeable batteries[J]. Chemical Communications, 2011, 47(41): 11474-11476. DOI: 10.1039/c1cc15320j. |
| 150 | BRAMNIK N N, BRAMNIK K G, BAEHTZ C, et al. Study of the effect of different synthesis routes on Li extraction-insertion from LiCoPO4[J]. Journal of Power Sources, 2005, 145(1): 74-81. DOI: 10.1016/j.jpowsour.2004.12.036. |
| 151 | EOM J, CHO J. M3(PO4)2-nanoparticle-coated LiCoO2 vs LiCo0.96M0.04O2(M=Mg and Zn) on electrochemical and storage characteristics[J]. Journal of the Electrochemical Society, 2008, 155(3): A201. DOI: 10.1149/1.2827993. |
| 152 | HU G R, DENG X R, PENG Z D, et al. Comparison of AlPO4- and Co3(PO4)2-coated LiNi0.8Co0.2O2 cathode materials for Li-ion battery[J]. Electrochimica Acta, 2008, 53(5): 2567-2573. DOI: 10.1016/j.electacta.2007.10.040. |
| 153 | ZHU Z, XU S L, WANG Z J, et al. Avoiding electrochemical indentations: A CNT-cocooned LiCoO2 electrode with ultra-stable high-voltage cycling[J]. Energy & Environmental Science, 2024, 17(16): 6102-6112. DOI: 10.1039/D4EE00722K. |
| 154 | LIU Z W, SHI L F, YUE K, et al. Stalling CO2 evolution at high voltage by a catalytically induced LiF-rich interphase[J]. Materials Today, 2025, 85: 69-81. DOI: 10.1016/j.mattod. 2025.02.017. |
| 155 | YASUHARA S, YASUI S, TERANISHI T, et al. Enhancement of ultrahigh rate chargeability by interfacial nanodot BaTiO3 treatment on LiCoO2 cathode thin film batteries[J]. Nano Letters, 2019, 19(3): 1688-1694. DOI: 10.1021/acs.nanolett. 8b04690. |
| 156 | CAI M Z, DONG Y H, XIE M, et al. Stalling oxygen evolution in high-voltage cathodes by lanthurization[J]. Nature Energy, 2023, 8(2): 159-168. DOI: 10.1038/s41560-022-01179-3. |
| 157 | LI Z J, YI H C, REN H Y, et al. Multiple surface optimizations for a highly durable LiCoO2 beyond 4.6 V[J]. Advanced Functional Materials, 2023, 33(46): 2307913. DOI: 10.1002/adfm.202307913. |
| 158 | ZHANG W, CHENG F Y, WANG M, et al. Collective surface enabling an ultralong life of LiCoO2 at high voltage and elevated temperature[J]. Advanced Functional Materials, 2023, 33(42): 2304008. DOI: 10.1002/adfm.202304008. |
| 159 | HUANG W Y, LI J Y, ZHAO Q H, et al. Mechanochemically robust LiCoO2 with ultrahigh capacity and prolonged cyclability[J]. Advanced Materials, 2024, 36(32): 2405519. DOI: 10.1002/adma.202405519. |
| 160 | ZHANG M, HUANG W Y, TANG J Y, et al. Precise synthesis of 4.75 V-tolerant LiCoO2 with homogeneous delithiation and reduced internal strain[J]. Journal of the American Chemical Society, 2025, 147(2): 1563-1573. DOI: 10.1021/jacs.4c10976. |
| 161 | KIM S, LEE J A, LEE D G, et al. Designing electrolytes for stable operation of high-voltage LiCoO2 in lithium-ion batteries[J]. ACS Energy Letters, 2024, 9(1): 262-270. DOI: 10.1021/acsenergylett.3c02534. |
| 162 | LUO H Y, JI X Y, ZHANG B D, et al. Revealing the dynamic evolution of electrolyte configuration on the cathode-electrolyte interface by visualizing (de) solvation processes[J]. Angewandte Chemie International Edition, 2024, 63(51): e202412214. DOI: 10.1002/anie.202412214. |
| 163 | YAN Y W, WENG S T, FU A, et al. Tailoring electrolyte dehydrogenation with trace additives: Stabilizing the LiCoO2 cathode beyond 4.6 V[J]. ACS Energy Letters, 2022, 7(8): 2677-2684. DOI: 10.1021/acsenergylett.2c01433. |
| 164 | ZHANG A P, BI Z H, WANG G R, et al. Regulating electrode/electrolyte interfacial chemistry enables 4.6 V ultra-stable fast charging of commercial LiCoO2[J]. Energy & Environmental Science, 2024, 17(9): 3021-3031. DOI: 10.1039/D4EE00676C. |
| 165 | YANG H Y, ZHAO Y J, QIN T, et al. Chemically active sulfonate additive with transition metal and oxygen dual-site deactivation for high-voltage LiCoO2[J]. ACS Energy Letters, 2024, 9(9): 4475-4484. DOI: 10.1021/acsenergylett.4c01898. |
| 166 | ZHANG H, HUANG Y X, WANG Y, et al. In-situ constructed protective bilayer enabling stable cycling of LiCoO2 cathode at high-voltage[J]. Energy Storage Materials, 2023, 62: 102951. DOI: 10.1016/j.ensm.2023.102951. |
| 167 | WU D X, ZHU C L, WANG H P, et al. Mechanically and thermally stable cathode electrolyte interphase enables high-temperature, high-voltage Li||LiCoO2 batteries[J]. Angewandte Chemie International Edition, 2024, 63(7): e202315608. DOI: 10.1002/anie.202315608. |
| 168 | SUN Z Y, ZHOU H B, LUO X H, et al. Design of a novel electrolyte additive for high voltage LiCoO2 cathode lithium-ion batteries: Lithium 4-benzonitrile trimethyl borate[J]. Journal of Power Sources, 2021, 503: 230033. DOI: 10.1016/j.jpowsour. 2021.230033. |
| 169 | LI Z J, ZHAO W G, REN H Y, et al. Tuning surface reconfiguration for durable cathode/electrolyte interphase of LiCoO2 at 45 ℃[J]. Advanced Energy Materials, 2024, 14(46): 2402223. DOI: 10.1002/aenm.202402223. |
| 170 | REN H Y, HU J X, JI H C, et al. Densification of cathode/electrolyte interphase to enhance reversibility of LiCoO2 at 4.65 V[J]. Advanced Materials, 2024, 36(40): 2408875. DOI: 10.1002/adma.202408875. |
| 171 | REN H Y, ZHENG G R, LI Y H, et al. Stabilizing LiCoO2 at 4.6 V by regulating anti-oxidative solvents[J]. Energy & Environmental Science, 2024, 17(20): 7944-7957. DOI: 10.1039/D4EE02049A. |
| 172 | SRIVASTAVA I, BOLINTINEANU D S, LECHMAN J B, et al. Controlling binder adhesion to impact electrode mesostructures and transport[J]. ACS Applied Materials & Interfaces, 2020, 12(31): 34919-34930. DOI: 10.1021/acsami.0c08251. |
| 173 | CAO Y L, CAI H S, XU X, et al. Fucoidan cross-linking polyacrylamide as multifunctional aqueous binder stabilizes LiCoO2 to 4.6 V[J]. Advanced Functional Materials, 2024, 34(40): 2405911. DOI: 10.1002/adfm.202405911. |
| 174 | 高桂红, 刘福园, 李珅珅, 等. 二元导电剂对锂浆料电池性能的影响[J]. 储能科学与技术, 2023, 12(11): 3299-3306. DOI: 10.19799/j.cnki.2095-4239.2023.0505. |
| GAO G H, LIU F Y, LI S S, et al. Effect of binary composite conductive agent on the performance of lithium slurry battery[J]. Energy Storage Science and Technology, 2023, 12(11): 3299-3306. DOI: 10.19799/j.cnki.2095-4239.2023.0505. | |
| 175 | WU X S, XIA S X, HUANG Y Q, et al. High-performance, low-cost, and dense-structure electrodes with high mass loading for lithium-ion batteries[J]. Advanced Functional Materials, 2019, 29(34): 1903961. DOI: 10.1002/adfm.201903961. |
| 176 | CHEN C J, ZHANG Y, LI Y J, et al. Highly conductive, lightweight, low-tortuosity carbon frameworks as ultrathick 3D current collectors[J]. Advanced Energy Materials, 2017, 7(17): 1700595. DOI: 10.1002/aenm.201700595. |
| 177 | ZHENG J X, GARCIA-MENDEZ R, ARCHER L A. Engineering multiscale coupled electron/ion transport in battery electrodes[J]. ACS Nano, 2021, 15(12): 19014-19025. DOI: 10.1021/acsnano.1c08719. |
| 178 | SHI Y, WEN L, PEI S F, et al. Choice for graphene as conductive additive for cathode of lithium-ion batteries[J]. Journal of Energy Chemistry, 2019, 30: 19-26. DOI: 10.1016/j.jechem.2018.03.009. |
| 179 | YAN L J, WANG K, LUO S, et al. Sandwich-structured cathodes with cross-stacked carbon nanotube films as conductive layers for high-performance lithium-ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(8): 4047-4057. DOI: 10.1039/C6TA10024D. |
| 180 | USSEGLIO-VIRETTA F L E, MAI W, COLCLASURE A M, et al. Enabling fast charging of lithium-ion batteries through secondary-/dual-pore network: Part I—Analytical diffusion model[J]. Electrochimica Acta, 2020, 342: 136034. DOI: 10.1016/j.electacta.2020.136034. |
| 181 | ZHANG X, HUI Z Y, KING S, et al. Tunable porous electrode architectures for enhanced Li-ion storage kinetics in thick electrodes[J]. Nano Letters, 2021, 21(13): 5896-5904. DOI: 10.1021/acs.nanolett.1c02142. |
| 182 | HUANG H, LI Z Q, GU S, et al. Dextran sulfate lithium as versatile binder to stabilize high-voltage LiCoO2 to 4.6 V[J]. Advanced Energy Materials, 2021, 11(44): 2101864. DOI: 10.1002/aenm.202101864. |
| 183 | LI S P, XIONG R Y, HAN Z L, et al. Unveiling low-tortuous effect on electrochemical performance toward ultrathick LiFePO4 electrode with 100 mg·cm-2 area loading[J]. Journal of Power Sources, 2021, 515: 230588. DOI: 10.1016/j.jpowsour.2021.230588. |
| 184 | HE R J, TIAN G L, LI S P, et al. Enhancing the reversibility of lithium cobalt oxide phase transition in thick electrode via low tortuosity design[J]. Nano Letters, 2022, 22(6): 2429-2436. DOI: 10.1021/acs.nanolett.2c00123. |
| 185 | ZHU C B, USISKIN R E, YU Y, et al. The nanoscale circuitry of battery electrodes[J]. Science, 2017, 358(6369): eaao2808. DOI: 10.1126/science.aao2808. |
| 186 | LIU Z M, ZENG Y Q, TAN J Y, et al. Revealing the degradation pathways of layered Li-rich oxide cathodes[J]. Nature Nanotechnology, 2024, 19(12): 1821-1830. DOI: 10.1038/s41565-024-01773-4. |
| [1] | Jiahui LIU, Weixiang BIAN, Dawei LI. In situ measurement and analysis of the electromechanical coupling performance of composite graphite electrodes in lithium batteries [J]. Energy Storage Science and Technology, 2025, 14(6): 2240-2247. |
| [2] | Chunling WU, Liding WANG, Yong LU, Limin GENG, Hao CHEN, Jinhao MENG. Lithium-ion batteries SOH estimation based on gaussian processed regression optimized by egret swarm optimization [J]. Energy Storage Science and Technology, 2025, 14(6): 2498-2511. |
| [3] | Haiyang ZHOU, Zhendong ZHANG, Lei SHENG, Zehua ZHU, Xiaojun ZHANG, Chunfeng ZHANG. Simulation of immersion thermal performance regulation and thermal safety experimental study for energy storage lithium batteries [J]. Energy Storage Science and Technology, 2025, 14(5): 1866-1874. |
| [4] | Zhoulan ZENG, Lei SHANG, Zhijin HU, Zongfan WANG, Xiaochao XIN, Ying LIU. Li5FeO4@C high capacity prelithium cathode materials for lithium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(5): 1875-1883. |
| [5] | Ziming MO, Zongxin RAO, Jianfei YANG, Menghao YANG, Liming CAI. Construction and characteristic analysis of key parameters in a gas-thermal model for thermal runaway in lithium-ion battery based on overcharge [J]. Energy Storage Science and Technology, 2025, 14(5): 1784-1796. |
| [6] | Lei PENG, Zhaopeng NI, Yue YU, Fupeng SUN, Xiulong XIA, Peng ZHANG, Sibo SUN. Experimental study on NCM lithium-ion battery electric vehicle fire caused by overcharging [J]. Energy Storage Science and Technology, 2025, 14(4): 1484-1495. |
| [7] | Jiangwei SHEN, Yixin SHE, Xing SHU, Yonggang LIU, Fuxing WEI, Xuelei XIA, Zheng CHEN. State of health estimation for lithium batteries based on short-term random charging data and optimized convolutional neural network [J]. Energy Storage Science and Technology, 2025, 14(4): 1585-1595. |
| [8] | Ruihao LIU, Xiaole MA, Yuxuan ZHANG, Yueying ZHU, Shiqiang LIU, Guangli BAI. Influencing factors of thermal property parameter testing of lithium-ion batteries based on accelerating rate calorimeters [J]. Energy Storage Science and Technology, 2025, 14(4): 1596-1602. |
| [9] | Zuolin DONG, Jinyan SONG, Zidi MENG. Lithium-ion battery life prediction based on mode decomposition and deep learning [J]. Energy Storage Science and Technology, 2025, 14(4): 1645-1653. |
| [10] | Zhiming CHEN, Aimin CHU, Ziyu ZHOU, Yuping Zhao, Youming CHEN. Preparation and performance of Li-rich cathode material by carbon-containing droplet combustion [J]. Energy Storage Science and Technology, 2025, 14(4): 1362-1368. |
| [11] | Jinming YUE, Yuanli LIU, Yixia CHEN, Xiqian YU, Hong LI. Study on the separation conditions of lithium ion battery electrolyte by GC-MS detection [J]. Energy Storage Science and Technology, 2025, 14(4): 1564-1573. |
| [12] | Xingqun LIAO, Rui YANG, Lijuan YU, Dalin HU, Feng XIAO, Jing HU, Zhouguang LU. 2,6-pyridine dimethyl acetonitrile: A multifunctional electrolyte additive for stabilizing high-voltage LiCoO2 [J]. Energy Storage Science and Technology, 2025, 14(4): 1331-1339. |
| [13] | Peng WANG, Jun ZHOU, Xing WU, Tao LIU. Remaining useful life prediction of a lithium-ion battery based on a cheetah optimization-extreme learning machine with improved Sine chaotic mapping [J]. Energy Storage Science and Technology, 2025, 14(4): 1603-1616. |
| [14] | Dequan HUANG, Tao WEI, Guangda YIN, Gang WEN, Jue HOU, Yi LIANG. Research on the application of siloxane solvent in high-voltage lithium metal batteries and electrochemical performance [J]. Energy Storage Science and Technology, 2025, 14(4): 1340-1351. |
| [15] | Shuaibo ZENG, Yongyi LI, Jing PENG, Zixing HE, Zhuojian LIANG, Wei XU, Lingxiao LAN, Xinghua LIANG. Optimization design of conductive agent based on ternary lithium-ion battery [J]. Energy Storage Science and Technology, 2025, 14(3): 1187-1197. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
