Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (10): 3715-3729.doi: 10.19799/j.cnki.2095-4239.2025.0252
• Energy Storage Materials and Devices • Previous Articles Next Articles
Yao LI1( ), Tianyang XUE1, Zhengjiao XIE3, Ji QIAN1,2,3(
), Tianyang XUE1, Zhengjiao XIE3, Ji QIAN1,2,3( ), Li LI1,2,3,4, Renjie CHEN1,2,3,4(
), Li LI1,2,3,4, Renjie CHEN1,2,3,4( )
)
Received:2025-03-16
Revised:2025-04-06
Online:2025-10-28
Published:2025-10-20
Contact:
Ji QIAN, Renjie CHEN
E-mail:liyao0029@163.com;jiqian@bit.edu.cn;chenrj@bit.edu.cn
CLC Number:
Yao LI, Tianyang XUE, Zhengjiao XIE, Ji QIAN, Li LI, Renjie CHEN. Low-temperature electrolyte optimization for lithium batteries: Challenges, advances, and multidimensional collaborative design[J]. Energy Storage Science and Technology, 2025, 14(10): 3715-3729.
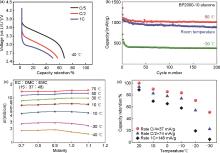
Fig. 1
(a) Discharge curves at -40 ℃ and three different discharge rates[17]; (b) Cycle performance operating at various temperatures[18]; (c) Ionic conductivity at different temperatures[19]; (d) Capacity retention ratio in respect to the steady state value of 115 mAh/g at the various currents and temperatures[20]"
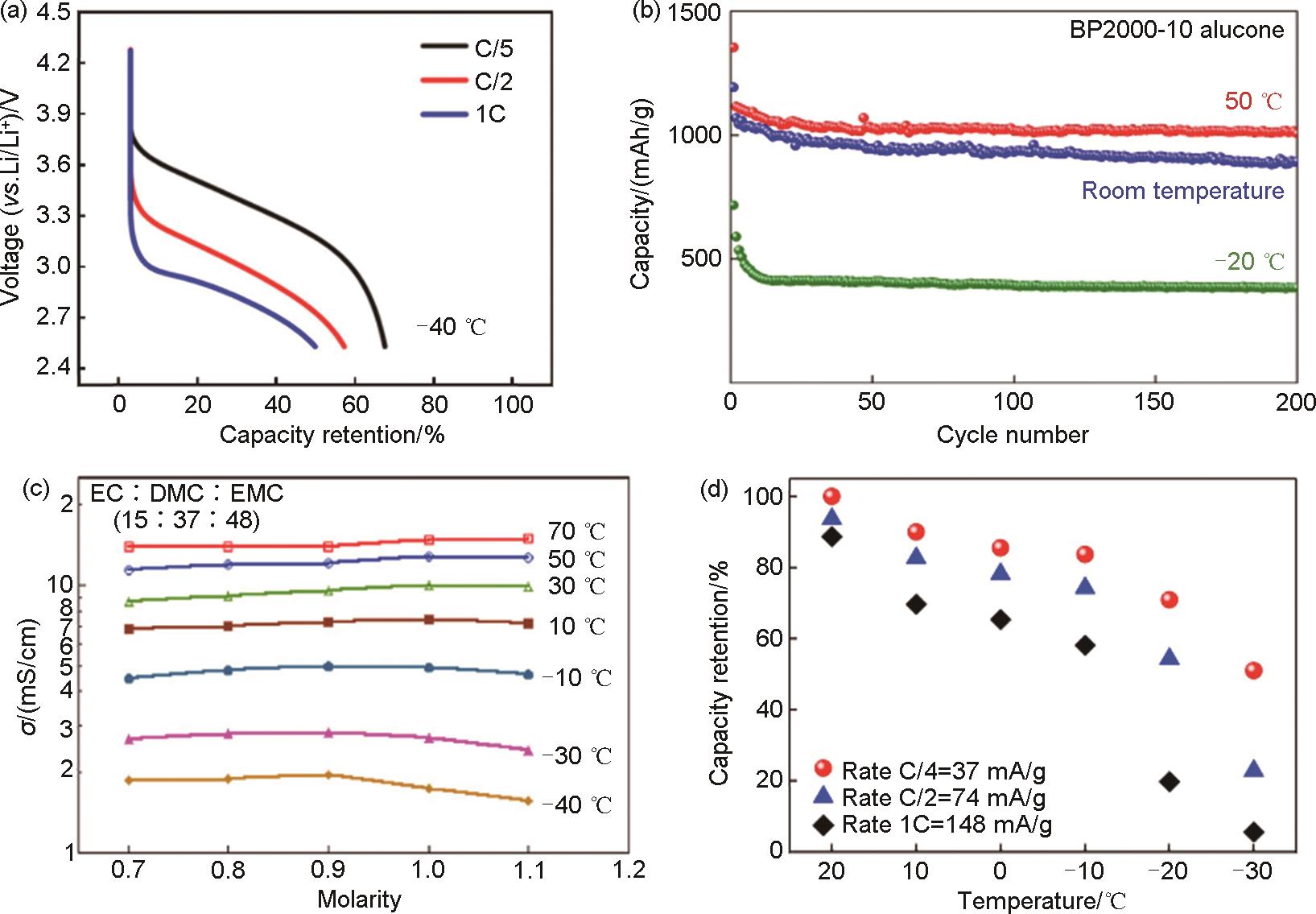
Fig. 2
(a) Solvation structure of COE and PM-2 electrolyte[22]; (b) Specific capacity-voltage diagram of PM-2 electrolyte over a wide temperature range[22]; (c) The illustration of the electron withdrawing effect of F atoms in the EA-2F solvent molecule and the corresponding coordination chemistry of the ethoxy side difluoro-substitution group (—OCH2CF2H)[23]; (d) Illustrations of fluorinated carbonate electrolytes. The transparent blue spheres indicate the solvation structure; (e) The different affinities between varied solvents and Li+; (f) The nonflammability and high electrochemical stability of designed electrolyte; (g) The expected electrochemical behaviors of tamed electrolyte at the electrode and electrolyte interface[24]"


Fig. 3
(a) The cycle performance of pouch cells in the E-control and dual-salt electrolytes at -20 ℃ at a charge rate of 4C and discharge rate of 0.5 C; (b) Interfacial stability and low-temperature performance of a 200 mAh Gr||NCM523 pouch cell. Cycle performance under -20 ℃ at 0.1C charge and 0.2C discharge[34]; Practicability of the dual-salt electrolyte for graphite||LCO pouch cells; (c) 2C rate under -20 ℃; (d) 1 Ah pouch cell cycle performance under -50 ℃[36]"
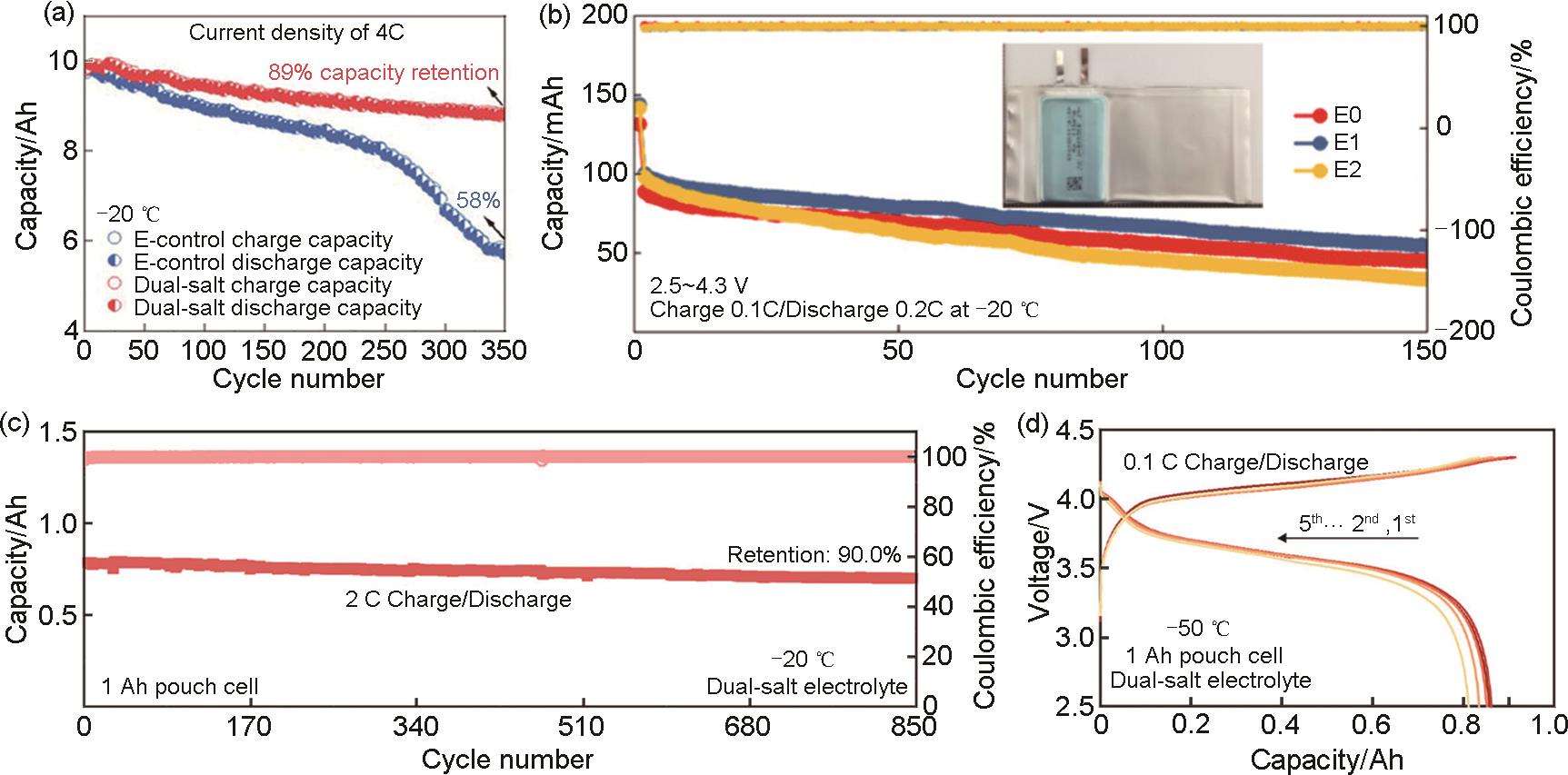

Fig. 4
(a) The temperature-dependent discharge profiles of Gr||NCM811 pouch cells with RT charge-LT discharge protocol[39]; (b) Charge-discharge curves of the pouch cells using the 1.5 mol/L MTOS electrolyte and commercial electrolyte at LT (0.1C)[39]; (c) Temperature dependence of ionic conductivities of electrolyte solutions adopting the additives[40]; (d) Temperature-dependent coulombic efficiency measurements at -60 ℃[41]; (e) Cycling performance of Li||NCM811 cells with THP/FEC at -40 ℃ and 4.5 V[42]"
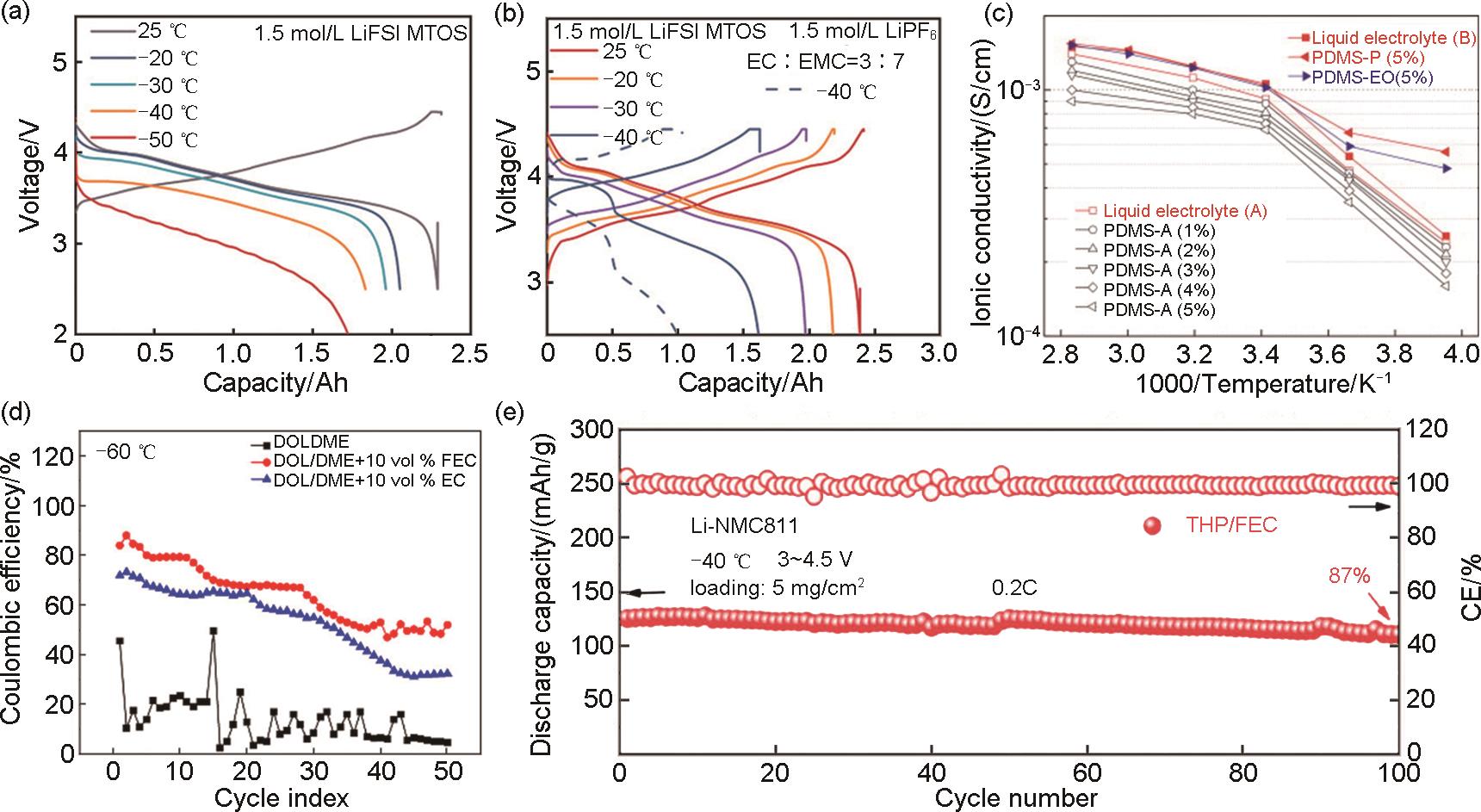
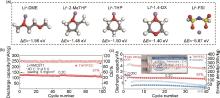
Fig. 6
(a) Different binding energies of Li+-DME、Li+-2-MeTHF、Li+-THP、Li+-1,4-DX and Li+-FSI-[51]; (b) Cycling performance of Li||NCM811 cells with THP/FEC at -40 ℃ and 4.5 V[42]; (c) Cycle performance of 2.7 Ah Li||NCM811 pouch ceels using THP/FEC and EC/DEC electrolytes at 20 ℃ and 4.5V[42]"
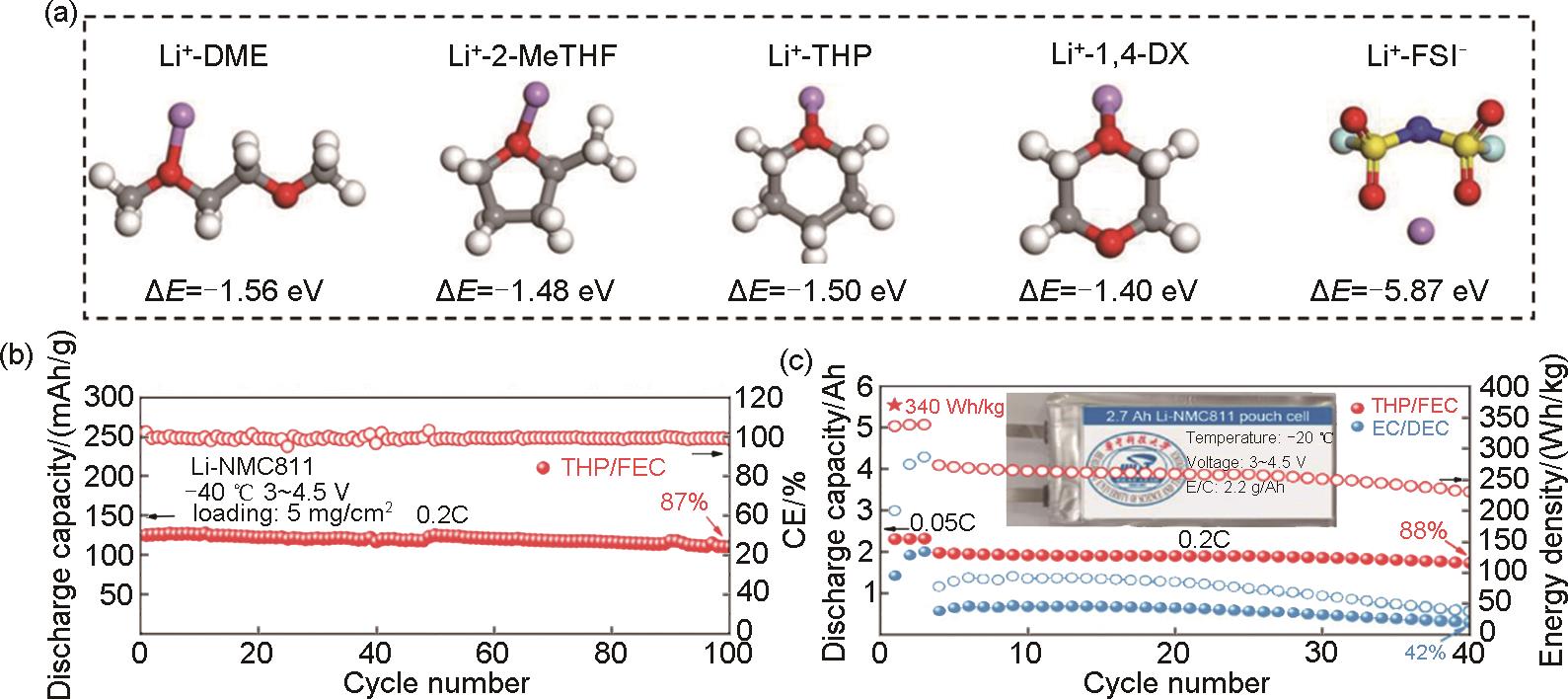

Fig. 8
(a) Diagram of a safe LPG electrolyte based on 1,1,1, 2-tetrafluoroethane and pentafluoroethane[59]; (b) The CE of Li-metal plating/strippingin various electrolytes at different temperatures[59]; (c) Measured ionic conductivities of the investigated electrolytes at different temperatures[60]; (d) Measured electrochemical performance at a wide-temperature range of 1 mol/L LiBF4-Me2O-PC[60]; (e) Ionic conductivity of liquefied gas electrolytes with different salt and cosolvent concentrations[61]"
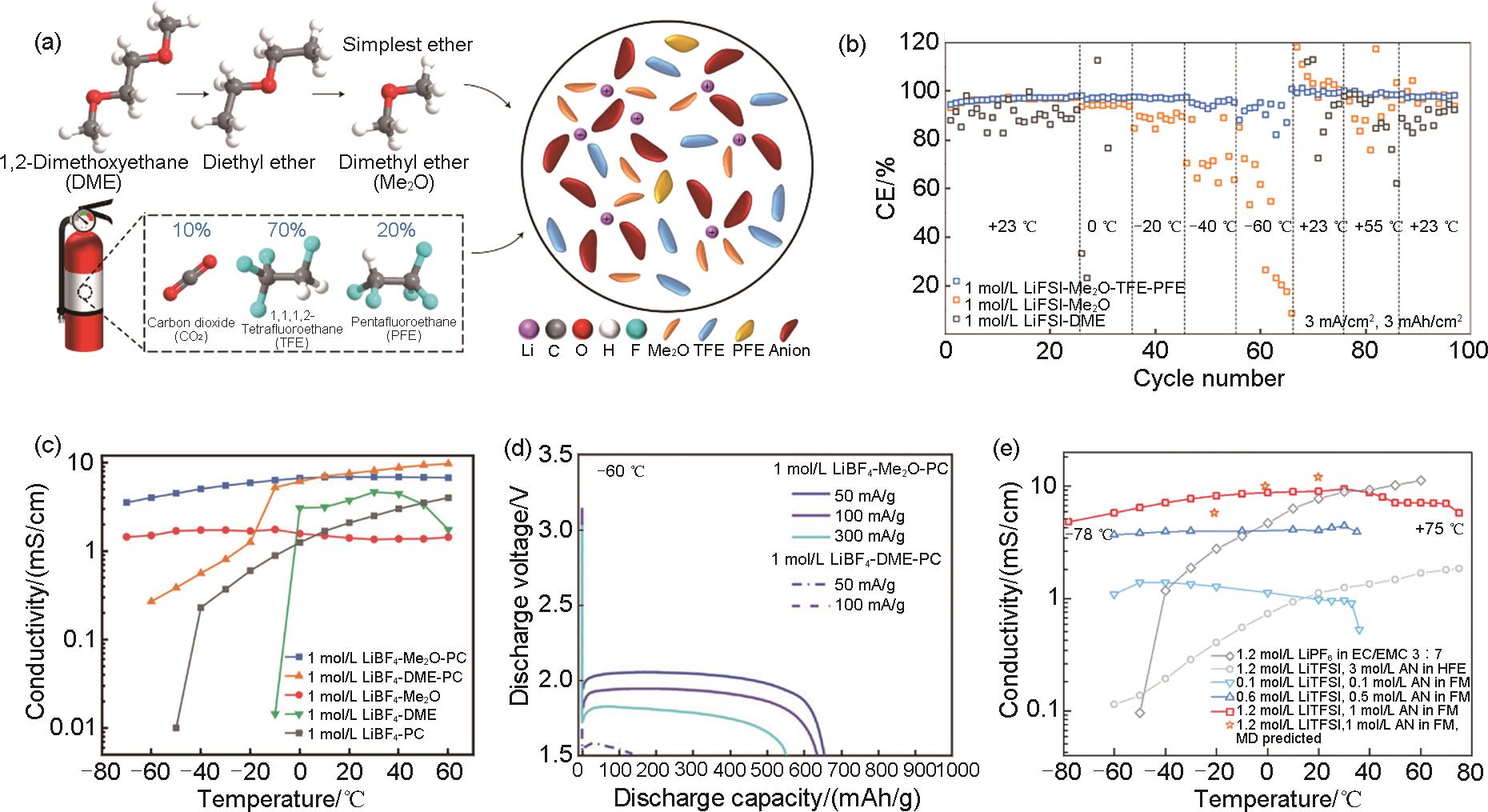
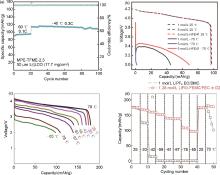
Fig. 9
(a) Cycling performance Li||LCO with lean Li inventory using MPE-TFME-2.3 electrolyte at -60 ℃ and -40 ℃[62]; (b) Discharge performance of Li||LMO cells at 0.1C at -70 ℃[63]; (c) Discharge profiles of Li||NCA cells using 1.28 mol/L LiFSI-FEC/FEMC-D2 electrolyte at different temperatures[24]; (d) Discharge capacities of Li||NCA cells using 1.28 mol/L LiFSI-FEC/FEMC-D2 and 1.0 mol/L LiPF6-EC/DMC electrolytes at different temperatures[24]"
Table 1
Performance parameters of different electrolyte systems"
| 电解液体系 | 离子电导率 | 容量保持率/% | 温度范围/℃ |
|---|---|---|---|
| 碳酸酯电解液 | -30 ℃: ~2.0 mS/cm | -20 ℃下保持95%左右的室温容量 | -20~55[ |
| 醚类电解液 | -40 ℃: 2~4 mS/cm | -40 ℃下保持66%左右的室温容量 | -70~60[ |
| 氟化腈类电解液 | 25 ℃: 40.3 mS/cm -70 ℃: 11.9 mS/cm | -80 ℃下保持51%左右的室温容量 | -80~60[ |
| 弱溶剂化电解液 | -50 ℃: 0.73 mS/cm | -40 ℃下保持87%左右的室温容量 | -60~105[ |
| 离子液体电解液 | -20 ℃: 1.67 mS/cm | -20 ℃下保持70%以上的室温容量 | -60~100[ |
| 液化气体电解液(LGE) | -70~60 ℃: >3.5 mS/cm | -60 ℃下保持91%左右的室温容量 | -78~80[ |
| 局部高浓电解液 | -80 ℃ >0.01 mS/cm | -85 ℃下保持56%的室温容量 | -80~70[ |
| [1] | EMBLEMSVÅG J. Wind energy is not sustainable when balanced by fossil energy[J]. Applied Energy, 2022, 305: 117748. DOI: 10.1016/j.apenergy.2021.117748. |
| [2] | LANDI B J, GANTER M J, CRESS C D, et al. Carbon nanotubes for lithium ion batteries[J]. Energy & Environmental Science, 2009, 2(6): 638-654. DOI: 10.1039/B904116H. |
| [3] | GHOSH A, SHUKLA S, MONISHA M, et al. Sulfur copolymer: A new cathode structure for room-temperature sodium-sulfur batteries[J]. ACS Energy Letters, 2015, 2: 2478-2485. DOI: 10.1021/ACSENERGYLETT.7B00714. |
| [4] | AHUJA R, BLOMQVIST A, LARSSON P, et al. Relativity and the lead-acid battery[J]. Physical Review Letters, 2011, 106(1): 018301. DOI: 10.1103/PhysRevLett.106.018301. |
| [5] | ZHANG M M, CHEN J Y, LI H, et al. Recent progress in Li-ion batteries with TiO2 nanotube anodes grown by electrochemical anodization[J]. Rare Metals, 2021, 40(2): 249-271. DOI: 10.1007/s12598-020-01499-x. |
| [6] | LIU Y, WANG Y, WANG F, et al. Facile synthesis of antimony tungstate nanosheets as anodes for lithium-ion batteries[J]. Nanomaterials, 2019, 9(12): 1689. DOI: 10.3390/nano9121689. |
| [7] | WU F X, YUSHIN G. Conversion cathodes for rechargeable lithium and lithium-ion batteries[J]. Energy & Environmental Science, 2017, 10(2): 435-459. DOI: 10.1039/C6EE02326F. |
| [8] | LIN D C, LIU Y Y, CUI Y. Reviving the lithium metal anode for high-energy batteries[J]. Nature Nanotechnology, 2017, 12(3): 194-206. DOI: 10.1038/nnano.2017.16. |
| [9] | ZHANG S S, XU K, JOW T R. Poly(acrylonitrile-methyl methacrylate) as a non-fluorinated binder for the graphite anode of Li-ion batteries[J]. Journal of Applied Electrochemistry, 2003, 33(11): 1099-1101. DOI: 10.1023/A:1026225001109. |
| [10] | VIDAL C, GROSS O, GU R, et al. xEV Li-ion battery low-temperature effects—Review[J]. IEEE Transactions on Vehicular Technology, 2019, 68(5): 4560-4572. DOI: 10.1109/TVT.2019.2906487. |
| [11] | RAMPAL N, WEITZNER S E, CHO S, et al. Structural and transport properties of battery electrolytes at sub-zero temperatures[J]. Energy & Environmental Science, 2024, 17(20): 7691-7698. DOI: 10.1039/D4EE01437E. |
| [12] | QIN M S, ZENG Z Q, CHENG S J, et al. Challenges and strategies of formulating low-temperature electrolytes in lithium-ion batteries[J]. Interdisciplinary Materials, 2023, 2(2): 308-336. DOI: 10.1002/idm2.12077. |
| [13] | THENUWARA A C, SHETTY P P, MCDOWELL M T. Distinct nanoscale interphases and morphology of lithium metal electrodes operating at low temperatures[J]. Nano Letters, 2019, 19(12): 8664-8672. DOI: 10.1021/acs.nanolett.9b03330. |
| [14] | CHEN S Y, MENG X D, HAN D J, et al. A covalently bonded, LiF-rich solid electrolyte interphase for Li metal batteries with superior low-temperature performance[J]. Chemical Engineering Journal, 2024, 500: 156909. DOI: 10.1016/j.cej.2024.156909. |
| [15] | WENG S T, ZHANG X, YANG G J, et al. Temperature-dependent interphase formation and Li+ transport in lithium metal batteries[J]. Nature Communications, 2023, 14: 4474. DOI: 10.1038/s41467-023-40221-0. |
| [16] | QIAN Y X, CHU Y L, ZHENG Z T, et al. A new cyclic carbonate enables high power/low temperature lithium-ion batteries[J]. Energy Storage Materials, 2022, 45: 14-23. DOI: 10.1016/j.ensm. 2021.11.029. |
| [17] | LI Q Y, JIAO S H, LUO L L, et al. Wide-temperature electrolytes for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(22): 18826-18835. DOI: 10.1021/acsami.7b04099. |
| [18] | LI X, BANIS M, LUSHINGTON A, et al. A high-energy sulfur cathode in carbonate electrolyte by eliminating polysulfides via solid-phase lithium-sulfur transformation[J]. Nature Communications, 2018, 9: 4509. DOI: 10.1038/s41467-018-06877-9. |
| [19] | MANDAL B K, PADHI A K, SHI Z, et al. New low temperature electrolytes with thermal runaway inhibition for lithium-ion rechargeable batteries[J]. Journal of Power Sources, 2006, 162(1): 690-695. DOI: 10.1016/j.jpowsour.2006.06.053. |
| [20] | ELIA G A, NOBILI F, TOSSICI R, et al. Nanostructured tin-carbon/LiNi0.5Mn1.5O4 lithium-ion battery operating at low temperature[J]. Journal of Power Sources, 2015, 275: 227-233. DOI: 10.1016/j.jpowsour.2014.10.144. |
| [21] | SMART M C, RATNAKUMAR B V, CHIN K B, et al. Lithium-ion electrolytes containing ester cosolvents for improved low temperature performance[J]. Journal of the Electrochemical Society, 2010, 157(12): A1361. DOI: 10.1149/1.3501236. |
| [22] | FANG Z, YANG Y, ZHENG T L, et al. An all-climate CFx/Li battery with mechanism-guided electrolyte[J]. Energy Storage Materials, 2021, 42: 477-483. DOI: 10.1016/j.ensm.2021.08.002. |
| [23] | WEI Y, WANG H, LIN X, et al. Moderate solvation structures of lithium ions for high-voltage lithium metal batteries at -40 ℃[J]. Energy & Environmental Science, 2025, 18(2): 786-798. DOI: 10.1039/D4EE03192J. |
| [24] | FAN X L, JI X, CHEN L, et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents[J]. Nature Energy, 2019, 4(10): 882-890. DOI: 10.1038/s41560-019-0474-3. |
| [25] | DU L Y, WANG H M, YANG M, et al. Free-standing nanostructured architecture as a promising platform for high-performance lithium-sulfur batteries[J]. Small Structures, 2020, 1(3): 2000047. DOI: 10.1002/sstr.202000047. |
| [26] | JIN Q, QI X Q, YANG F Y, et al. The failure mechanism of lithium-sulfur batteries under lean-ether-electrolyte conditions[J]. Energy Storage Materials, 2021, 38: 255-261. DOI: 10.1016/j.ensm.2021. 03.014. |
| [27] | PENG H J, HUANG J Q, CHENG X B, et al. Review on high-loading and high-energy lithium-sulfur batteries[J]. Advanced Energy Materials, 2017, 7(24): 1700260. DOI: 10.1002/aenm. 201700260. |
| [28] | SHEN X, LIU H, CHENG X B, et al. Beyond lithium ion batteries: Higher energy density battery systems based on lithium metal anodes[J]. Energy Storage Materials, 2018, 12: 161-175. DOI: 10.1016/j.ensm.2017.12.002. |
| [29] | MIKHAYLIK Y V, AKRIDGE J R. Low temperature performance of Li/S batteries[J]. Journal of the Electrochemical Society, 2003, 150(3): A306. DOI: 10.1149/1.1545452. |
| [30] | LU D, LI R H, RAHMAN M M, et al. Ligand-channel-enabled ultrafast Li-ion conduction[J]. Nature, 2024, 627(8002): 101-107. DOI: 10.1038/s41586-024-07045-4. |
| [31] | MIN X Q, WANG L, WU Y Z, et al. Overcoming low-temperature challenges in LIBs: The role of anion-rich solvation sheath in strong solvents[J]. Journal of Energy Chemistry, 2025, 106: 63-70. DOI: 10.1016/j.jechem.2025.02.027. |
| [32] | LIU X, ZHANG J W, LI J, et al. Steric coordinated electrolytes for fast-charging and low-temperature energy-dense lithium-ion batteries[J]. Angewandte Chemie International Edition, 2025, 64(23): e202502978. DOI: 10.1002/anie.202502978. |
| [33] | WANG L, YU F D, QUE L F, et al. 12-Ah-level Li-ion pouch cells enabling fast charging at temperatures between -20 and 50 ℃[J]. Advanced Functional Materials, 2024, 34(48): 2408422. DOI: 10.1002/adfm.202408422. |
| [34] | QIN N, CHEN J, LU Y Y, et al. Trace LiBF4 enabling robust LiF-rich interphase for durable low-temperature lithium-ion pouch cells[J]. ACS Energy Letters, 2024, 9(10): 4843-4851. DOI: 10.1021/acsenergylett.4c01616. |
| [35] | FENG M, CHEN N, CHEN R. Research progress of low-temperature electrolyte for lithium-ion battery [J]. Energy Storage Science and Technology, 2023, 12(3): 792. |
| [36] | ZHAO Y M, HU Z L, ZHAO Z F, et al. Strong solvent and dual lithium salts enable fast-charging lithium-ion batteries operating from -78 to 60 ℃[J]. Journal of the American Chemical Society, 2023, 145(40): 22184-22193. DOI: 10.1021/jacs.3c08313. |
| [37] | SHANGGUAN X H, XU G J, CUI Z L, et al. Additive-assisted novel dual-salt electrolyte addresses wide temperature operation of lithium-metal batteries[J]. Small, 2019, 15(16): 1900269. DOI: 10.1002/smll.201900269. |
| [38] | ZHANG S S. A review on electrolyte additives for lithium-ion batteries[J]. Journal of Power Sources, 2006, 162(2): 1379-1394. DOI: 10.1016/j.jpowsour.2006.07.074. |
| [39] | WANG Y W, LIU J, JI H Q, et al. Optimizing Si─O conjugation to enhance interfacial kinetics for low-temperature rechargeable lithium-ion batteries[J]. Advanced Materials, 2025, 37(3): 2412155. DOI: 10.1002/adma.202412155. |
| [40] | KIM K M, LY N V, WON J H, et al. Improvement of lithium-ion battery performance at low temperature by adopting polydimethylsiloxane-based electrolyte additives[J]. Electrochimica Acta, 2014, 136: 182-188. DOI: 10.1016/j.electacta.2014.05.054. |
| [41] | THENUWARA A C, SHETTY P P, KONDEKAR N, et al. Efficient low-temperature cycling of lithium metal anodes by tailoring the solid-electrolyte interphase[J]. ACS Energy Letters, 2020, 5(7): 2411-2420. DOI: 10.1021/acsenergylett.0c01209. |
| [42] | LI Z Z, LIAO Y Q, JI H J, et al. A tetrahydropyran-based weakly solvating electrolyte for low-temperature and high-voltage lithium metal batteries[J]. Advanced Energy Materials, 2025, 15(15): 2404120. DOI: 10.1002/aenm.202404120. |
| [43] | SHI J L, EHTESHAMI N, MA J L, et al. Improving the graphite/electrolyte interface in lithium-ion battery for fast charging and low temperature operation: Fluorosulfonyl isocyanate as electrolyte additive[J]. Journal of Power Sources, 2019, 429: 67-74. DOI: 10.1016/j.jpowsour.2019.04.113. |
| [44] | GAO J X, HAN S Y, HUA H M, et al. Phenyl TrifluoroMethane sulfonate as a novel electrolyte additive for enhancing performance of LiNi0·6Co0·2Mn0·2O2/Graphite cells working in wide temperature ranges[J]. Journal of Power Sources, 2021, 487: 229416. DOI: 10.1016/j.jpowsour.2020.229416. |
| [45] | LIN Y C, YUE X P, ZHANG H, et al. Using phenyl methanesulfonate as an electrolyte additive to improve performance of LiNi0.5Co0.2Mn0.3O2/graphite cells at low temperature[J]. Electrochimica Acta, 2019, 300: 202-207. DOI: 10.1016/j.electacta.2019.01.120. |
| [46] | LIAO L X, FANG T, ZHOU X G, et al. Enhancement of low-temperature performance of LiFePO4 electrode by butyl sultone as electrolyte additive[J]. Solid State Ionics, 2014, 254: 27-31. DOI: 10.1016/j.ssi.2013.10.047. |
| [47] | JURNG S, PARK S, YOON T, et al. Low-temperature performance improvement of graphite electrode by allyl sulfide additive and its film-forming mechanism[J]. Journal of the Electrochemical Society, 2016, 163(8): A1798-A1804. DOI: 10.1149/2.0051609jes. |
| [48] | WU Z L, LI S G, ZHENG Y Z, et al. The roles of sulfur-containing additives and their working mechanism on the temperature-dependent performances of Li-ion batteries[J]. Journal of the Electrochemical Society, 2018, 165(11): A2792-A2800. DOI: 10. 1149/2.0331811jes. |
| [49] | WANG Z J, ZHANG B. Weakly solvating electrolytes for next-generation lithium batteries: Design principles and recent advances[J]. Energy Materials and Devices, 2023, 1(1): 9370003. DOI: 10.26599/emd.2023.9370003. |
| [50] | MA T, NI Y X, WANG Q R, et al. Optimize lithium deposition at low temperature by weakly solvating power solvent[J]. Angewandte Chemie, 2022, 134(39): e202207927. DOI: 10.1002/ange.202207927. |
| [51] | LIAO Y Q, ZHOU M Y, YUAN L X, et al. Eco-friendly tetrahydropyran enables weakly solvating "4S" electrolytes for lithium-metal batteries[J]. Advanced Energy Materials, 2023, 13(32): 2301477. DOI: 10.1002/aenm.202301477. |
| [52] | ZHOU Q, BOYLE P D, MALPEZZI L, et al. Phase behavior of ionic liquid-LiX mixtures: Pyrrolidinium cations and TFSI– anions-linking structure to transport properties[J]. Chemistry of Materials, 2011, 23(19): 4331-4337. DOI: 10.1021/cm201427k. |
| [53] | TSAI W Y, LIN R Y, MURALI S, et al. Outstanding performance of activated graphene based supercapacitors in ionic liquid electrolyte from -50 to 80 ℃[J]. Nano Energy, 2013, 2(3): 403-411. DOI: 10.1016/j.nanoen.2012.11.006. |
| [54] | ZHAO Q, CHEN P Y, LI S K, et al. Solid-state polymer electrolytes stabilized by task-specific salt additives[J]. Journal of Materials Chemistry A, 2019, 7(13): 7823-7830. DOI: 10.1039/C8TA12008K. |
| [55] | TIAN J R, CUI C J, XIE Q, et al. EMIMBF4-GBL binary electrolyte working at -70 ℃ and 3.7 V for a high performance graphene-based capacitor[J]. Journal of Materials Chemistry A, 2018, 6(8): 3593-3601. DOI: 10.1039/c7ta10474j. |
| [56] | XU Y F, LIN W J, GLIEGE M, et al. A dual ionic liquid-based low-temperature electrolyte system[J]. The Journal of Physical Chemistry B, 2018, 122(50): 12077-12086. DOI: 10.1021/acs.jpcb.8b08815. |
| [57] | WANG Z C, SUN Y Y, MAO Y Y, et al. Highly concentrated dual-anion electrolyte for non-flammable high-voltage Li-metal batteries[J]. Energy Storage Materials, 2020, 30: 228-237. DOI: 10.1016/j.ensm.2020.05.020. |
| [58] | LIN R Y, TABERNA P L, FANTINI S, et al. Capacitive energy storage from –50 to 100 ℃ using an ionic liquid electrolyte[J]. The Journal of Physical Chemistry Letters, 2011, 2(19): 2396-2401. DOI: 10.1021/jz201065t. |
| [59] | YIN Y J, YANG Y, CHENG D Y, et al. Fire-extinguishing, recyclable liquefied gas electrolytes for temperature-resilient lithium-metal batteries[J]. Nature Energy, 2022, 7(6): 548-559. DOI: 10.1038/s41560-022-01051-4. |
| [60] | YIN Y J, HOLOUBEK J, LIU A, et al. Ultralow-temperature Li/CFx batteries enabled by fast-transport and anion-pairing liquefied gas electrolytes[J]. Advanced Materials, 2023, 35(3): 2207932. DOI: 10.1002/adma.202207932. |
| [61] | YANG Y, YIN Y, DAVIES D M, et al. Liquefied gas electrolytes for wide-temperature lithium metalbatteries [J]. Energy & Environmental Science, 2020, 13(7): 2209-19. |
| [62] | ZHAO Z F, WANG A X, CHEN A S, et al. Leveraging ion pairing and transport in localized high-concentration electrolytes for reversible lithium metal anodes at low temperatures[J]. Angewandte Chemie International Edition, 2024, 63(45): e202412239. DOI: 10.1002/anie.202412239. |
| [63] | LAI P B, DENG X D, ZHANG Y Q, et al. Bifunctional localized high-concentration electrolyte for the fast kinetics of lithium batteries at low temperatures[J]. ACS Applied Materials & Interfaces, 2023, 15(25): 31020-31031. DOI: 10.1021/acsami.3c04747. |
| [64] | LU Z, LIU D, DAI K, et al. Tailoring solvation chemistry in carbonate electrolytes for all-climate, high-voltage lithium-rich batteries[J]. Energy Storage Materials, 2023, 57: 316-325. DOI: 10.1016/j.ensm.2023.02.029. |
| [65] | JIN C B, YAO N, XIAO Y, et al. Taming solvent-solute interaction accelerates interfacial kinetics in low-temperature lithium-metal batteries[J]. Advanced Materials, 2023, 35(3): 2208340. DOI: 10. 1002/adma.202208340. |
| [66] | LIU X, MARIANI A, DIEMANT T, et al. Locally concentrated ionic liquid electrolytes enabling low-temperature lithium metal batteries[J]. Angewandte Chemie International Edition, 2023, 62(31): e202305840. DOI: 10.1002/anie.202305840. |
| [1] | Zheng CHEN, Jingyuan HU, Zhigang ZHAO, Jiangwei SHEN, Xuelei XIA, Fuxing WEI. Thermal characteristics study and optimization of air-cooling structures for dual-system battery packs [J]. Energy Storage Science and Technology, 2025, 14(9): 3463-3475. |
| [2] | Tuo DENG, Haiping ZHOU, Yu LIU, Chang LIU, Zikai LI, Mengqiang WU. Research progress in the preparation of silicon-carbons anode by chemical vapor deposition [J]. Energy Storage Science and Technology, 2025, 14(9): 3354-3372. |
| [3] | Bin YANG, Jun YANG, Lang XU, Haowei WEN, Dengfeng LIU, Dianbo RUAN. Ball-head indentation-induced safety evaluation of capacitive lithium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(8): 3090-3099. |
| [4] | Chao PANG, Shuang DING, Xiaokun ZHANG, Yong XIANG. Simulation study of the solvation structure and ion migration behavior in localized high-concentration electrolytes [J]. Energy Storage Science and Technology, 2025, 14(8): 3207-3215. |
| [5] | Wei WANG, Huishi LIANG, Miangang LI, Kui ZHOU, Wei WANG, Ziyao WANG, Zinan SHI. Method for monitoring irreversible lithium plating in lithium batteries using transfer learning [J]. Energy Storage Science and Technology, 2025, 14(7): 2698-2706. |
| [6] | Zheng CHEN, Gongdong DUO, Jiangwei SHEN, Shiquan SHEN, Yu LIU, Fuxing WEI. State of health estimation for lithium battery based on incremental capacity analysis and VMD-GWO-KELM [J]. Energy Storage Science and Technology, 2025, 14(6): 2476-2487. |
| [7] | Jingjing RUAN, Xiangkun WU, Yonghui LI, Chongchong ZHAO, Shenshen LI, Tongfei WANG, Shengjie LIANG, Guihong GAO. Preparation and performance studies of low-cost graphite thick dry electrodes [J]. Energy Storage Science and Technology, 2025, 14(6): 2248-2255. |
| [8] | Dandan HAN, Wuwei ZHANG, Liang ZHANG, Zongjiang WANG. Design and electrochemical performance of LiMn1-y Fe y PO4/C cathode materials with a core-shell structure [J]. Energy Storage Science and Technology, 2025, 14(6): 2215-2222. |
| [9] | Yingjian CHEN, Shang WU, Yuancheng CAO, Baoshuai DU, Zhenxing WANG, Zhongwen OUYANG, Shun TANG. Application of magnetic separation in the recycling of cathode and anode materials from spent lithium batteries [J]. Energy Storage Science and Technology, 2025, 14(5): 1918-1927. |
| [10] | Xiaolan WU, Pengjie MA, Zhifeng BAI, Chenglong LIU, Guifang GUO, Jinhua ZHANG. A kind of intelligent PID double-layer active balancing control method for lithium-ion battery pack [J]. Energy Storage Science and Technology, 2025, 14(3): 1150-1159. |
| [11] | Nan LI, Jing MA, Tingxiu HUANG, Yixing SHEN, Min SHEN, Yiyi JIANG, Tao HONG, Guoqiang MA, Zifeng MA. Research progress on nitrile compounds in high potential electrolytes [J]. Energy Storage Science and Technology, 2025, 14(3): 997-1009. |
| [12] | Chencheng XU, Zhan WANG, Shuang LI, Jiangmin JIANG, Zhicheng JU. Research progress and engineering application prospects of prelithiation technology for lithium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(3): 930-946. |
| [13] | Liping ZHOU, Deqing ZHOU, Fenghua ZHENG, Qichang PAN, Sijiang HU, Yongjie JIANG, Hongqiang WANG, Qingyu LI. Preparation and application of Si@void@C composite anode materials for lithium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(3): 1115-1122. |
| [14] | Jiabo LI, Zhixuan WANG, Di TIAN, Zhonglin SUN. Prediction method for remaining service life of lithium batteries using SSA-LSTM combination under variable mode decomposition [J]. Energy Storage Science and Technology, 2025, 14(2): 659-670. |
| [15] | Jianru ZHANG, Qiyu WANG, Yinghui JI, Xin GAO, Xiqian YU, Hong LI. Application of Auger electron spectroscopy in the analysis of lithium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(2): 755-769. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
