Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (10): 3705-3714.doi: 10.19799/j.cnki.2095-4239.2025.0223
• Energy Storage Materials and Devices • Previous Articles Next Articles
Zhuoyan YI( ), Pengfei PANG, Yicong HUANG, Mingjie LIAO, Honghua LIANG, Guisheng ZHU(
), Pengfei PANG, Yicong HUANG, Mingjie LIAO, Honghua LIANG, Guisheng ZHU( ), Yunyun ZHAO, Huarui XU
), Yunyun ZHAO, Huarui XU
Received:2025-03-06
Revised:2025-04-13
Online:2025-10-28
Published:2025-10-20
Contact:
Guisheng ZHU
E-mail:3297395320@qq.com;zgs9539@163.com
CLC Number:
Zhuoyan YI, Pengfei PANG, Yicong HUANG, Mingjie LIAO, Honghua LIANG, Guisheng ZHU, Yunyun ZHAO, Huarui XU. Enhancing the electrochemical stability and ion transport performance of UV-Cured quasi-solid-state electrolytes via Al2O3 doping[J]. Energy Storage Science and Technology, 2025, 14(10): 3705-3714.
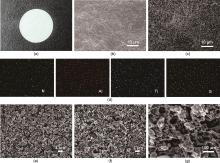
Fig. 2
(a) Photograph of OICSE-Al2O3-1; (b) Macroscopic SEM image of OICSE-Al2O3-0; (c) Macroscopic SEM image of OICSE-Al2O3-1; (d) Microscopic EDS mapping of OICSE-Al2O3-1; (e) Microscopic SEM image of OICSE-Al2O3-0; (f) Microscopic SEM image of OICSE-Al2O3-1; (g) High-magnification SEM image of OICSE-Al2O3-1"


Fig. 5
(a) Impedance spectra of OICSE-Al2O3-X at room temperature; (b) Lithium-ion transference number of OICSE-Al2O3-1 at room temperature; (c) LSV curves of OICSE-Al2O3-X; (d) Rate performance of Li||OICSE-Al2O3-0||LiFePO4 and Li||OICSE-Al2O3-1||LiFePO4 at 25 ℃; (e) Specific capacity-voltage curves of Li||OICSE-Al2O3-1||LiFePO4 at different rates at 25 ℃; (f) Ionic conductivity of OICSE-Al2O3-1 at different temperatures"

| [1] | LIU K, LIU Y Y, LIN D C, et al. Materials for lithium-ion battery safety[J]. Science Advances, 2018, 4(6): eaas9820. DOI: 10. 1126/sciadv.aas9820. |
| [2] | WU M H, HAN S H, LIU S M, et al. Fire-safe polymer electrolyte strategies for lithium batteries[J]. Energy Storage Materials, 2024, 66: 103174. DOI: 10.1016/j.ensm.2024.103174. |
| [3] | ZHANG W Q, NIE J H, LI F, et al. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane[J]. Nano Energy, 2018, 45: 413-419. DOI: 10.1016/j.nanoen.2018.01.028. |
| [4] | MANTHIRAM A, YU X W, WANG S F. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2: 16103. DOI: 10.1038/natrevmats.2016.103. |
| [5] | CHEN Q W, OUYANG C, LIANG Y T, et al. Composite polymer electrolyte with vertically aligned garnet scaffolds for quasi solid-state lithium batteries[J]. Energy Storage Materials, 2024, 69: 103418. DOI: 10.1016/j.ensm.2024.103418. |
| [6] | JIN Y M, ZONG X, ZHANG X B, et al. Constructing 3D Li+-percolated transport network in composite polymer electrolytes for rechargeable quasi-solid-state lithium batteries[J]. Energy Storage Materials, 2022, 49: 433-444. DOI: 10.1016/j.ensm. 2022.04.035. |
| [7] | JIAO K J, LIU S J, MA Y Y, et al. Long-term cycling quasi-solid-state lithium batteries enabled by 3D nanofibrous TiO2- x@Li anodes and in situ polymerized gel-electrolytes[J]. Chemical Engineering Journal, 2023, 464: 142627. DOI: 10.1016/j.cej. 2023.142627. |
| [8] | ZHANG X Y, CHENG S C, FU C K, et al. Advancements and challenges in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries[J]. Nano-Micro Letters, 2024, 17(1): 2. DOI: 10.1007/s40820-024-01498-y. |
| [9] | VU T T, CHEON H J, SHIN S Y, et al. Hybrid electrolytes for solid-state lithium batteries: Challenges, progress, and prospects[J]. Energy Storage Materials, 2023, 61: 102876. DOI: 10.1016/j.ensm.2023.102876. |
| [10] | LIU X Y, LI X R, LI H X, et al. Recent progress of hybrid solid-state electrolytes for lithium batteries[J]. Chemistry-A European Journal, 2018, 24(69): 18293-18306. DOI: 10.1002/chem.2018 03616. |
| [11] | CHEN L, ZHU F, MA D J, et al. Enhanced 3D framework composite solid electrolyte with alumina-modified Li1.4Al0.4Ti1.6(PO4)3 for solid-state lithium battery[J]. Ionics, 2024, 30(4): 2019-2028. DOI: 10.1007/s11581-024-05421-8. |
| [12] | GUO H L, SUN H, JIANG Z L, et al. A new type of composite electrolyte with high performance for room-temperature solid-state lithium battery[J]. Journal of Materials Science, 2019, 54(6): 4874-4883. DOI: 10.1007/s10853-018-03188-8. |
| [13] | MCOWEN D W, XU S M, GONG Y H, et al. 3D-printing electrolytes for solid-state batteries[J]. Advanced Materials, 2018, 30(18): 1707132. DOI: 10.1002/adma.201707132. |
| [14] | TANG K H, BAI Q S, XU P W, et al. A thiol branched 3D network quasi solid-state polymer electrolyte reinforced by covalent organic frameworks for lithium metal batteries[J]. Small Methods, 2024, 8(12): 2301810. DOI: 10.1002/smtd.202301810. |
| [15] | BAE M, AHN S, YOU S, et al. Expanded illite filler in UV-curable polymer electrolytes for all-solid-state Li-ion batteries[J]. Coatings, 2024, 14(9): 1158. DOI: 10.3390/coatings14091158. |
| [16] | ZHU T J, HAO X Q, CAO Y A, et al. Ultraviolet-cured heat-resistant and stretchable gel polymer electrolytes for flexible and safe semi-solid lithium-ion batteries[J]. Journal of Power Sources, 2024, 613: 234944. DOI: 10.1016/j.jpowsour.2024.234944. |
| [17] | WANG E L, LU Z Y, LIU C F, et al. UV curved PESF-LLZTO composite solid electrolyte to in situ construct ultrastable interface for all solid-state lithium battery[J]. Journal of the Electrochemical Society, 2024, 171(4): 040544. DOI: 10.1149/1945-7111/ad3eb8. |
| [18] | CHEN Z X, ZHANG Y Q, ZHU B S, et al. Construction of high-performance solid-state electrolytes for lithium metal batteries by UV-curing technology[J]. Polymer Testing, 2024, 132: 108386. DOI: 10.1016/j.polymertesting.2024.108386. |
| [19] | FAN H Y, YANG C H, WANG X D, et al. UV-curable PVDF-HFP-based gel electrolytes with semi-interpenetrating polymer network for dendrite-free lithium metal batteries[J]. Journal of Electroanalytical Chemistry, 2020, 871: 114308. DOI: 10.1016/j.jelechem.2020.114308. |
| [20] | JIN L, AHMED F, RYU T, et al. Highly conductive and flexible gel polymer electrolyte with bis(fluorosulfonyl)imide lithium salt via UV curing for Li-ion batteries[J]. Membranes, 2019, 9(11): 139. DOI: 10.3390/membranes9110139. |
| [21] | LU Y, HE K W, ZHANG S J, et al. UV-curable-based plastic crystal polymer electrolyte for high-performance all-solid-state Li-ion batteries[J]. Ionics, 2019, 25(4): 1607-1615. DOI: 10.1007/s11581-018-2788-8. |
| [22] | MASHEKOVA A, BALTASH Y, YEGAMKULOV M, et al. Polycationic doping of the LATP ceramic electrolyte for Li-ion batteries[J]. RSC Advances, 2022, 12(46): 29595-29601. DOI: 10.1039/D2RA05782D. |
| [23] | WANG S J, LV Q, JING Y T, et al. In situ polymerization design of a quasi-solid electrolyte enhanced by NMP additive for lithium metal batteries[J]. Energy Storage Materials, 2024, 69: 103390. DOI: 10.1016/j.ensm.2024.103390. |
| [24] | CAI B R, CAO J H, LIANG W H, et al. Ultraviolet-cured Al2O3-polyethylene terephthalate/polyvinylidene fluoride composite separator with asymmetric design and its performance in lithium batteries[J]. ACS Applied Energy Materials, 2021, 4(5): 5293-5303. DOI: 10.1021/acsaem.1c00804. |
| [25] | JI Y, ZHANG Y H, SHI F N, et al. UV-derived double crosslinked PEO-based solid polymer electrolyte for room temperature[J]. Journal of Colloid and Interface Science, 2023, 629: 492-500. DOI: 10.1016/j.jcis.2022.09.089. |
| [26] | WU X, JIE X H, LIANG X H, et al. Ultraviolet-thermal coupling cross-linked fabricate polymer/ceramic composite solid electrolyte for room temperature quasi solid state lithium ion batteries[J]. Journal of Energy Storage, 2024, 77: 109644. DOI: 10.1016/j.est. 2023.109644. |
| [27] | LI J Q, LIU C J, HE M Y, et al. Improved the electrochemical performance between ZnO@Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte and lithium metal electrode for all-solid-state lithium-ion batteries[J]. Electrochimica Acta, 2023, 439: 141549. DOI: 10.1016/j.electacta.2022.141549. |
| [28] | ZHOU P, ZHANG X K, XIANG Y, et al. Strategies to enhance Li+ transference number in liquid electrolytes for better lithium batteries[J]. Nano Research, 2023, 16(6): 8055-8071. DOI: 10. 1007/s12274-022-4833-1. |
| [1] | Wenyan CHEN, Ruilin HE, Jian CHANG, Yonghong DENG. Investigation of lithium storage mechanisms in liquid metal electrodes with different morphologies [J]. Energy Storage Science and Technology, 2025, 14(9): 3290-3300. |
| [2] | Honghui LIU, Donghui LI, Qifeng QIAN, Lingchao XIAO, Lei XIONG, Zhongguo CHEN. Preparation of vanadium nitride-based electrode materials and their application progress in supercapacitors [J]. Energy Storage Science and Technology, 2025, 14(8): 3110-3121. |
| [3] | Chen LIANG, Pengfei XING, Mengwu WU, Xunpeng QIN. Phase-field simulation study on the growth and dissolution of lithium dendrites during the charging and discharging processes of lithium metal batteries [J]. Energy Storage Science and Technology, 2025, 14(5): 1829-1840. |
| [4] | Yang LENG, Shuo HUANG, Kaixuan GUI, Wenqi YAN, Qi LIU. Study on polyanionic COFs-based composite separators for stabilizing aqueous zinc-ion battery anodes [J]. Energy Storage Science and Technology, 2025, 14(5): 1900-1909. |
| [5] | Dequan HUANG, Tao WEI, Guangda YIN, Gang WEN, Jue HOU, Yi LIANG. Research on the application of siloxane solvent in high-voltage lithium metal batteries and electrochemical performance [J]. Energy Storage Science and Technology, 2025, 14(4): 1340-1351. |
| [6] | Lei WANG, Shaomian LIU, Fenglan FAN, Ziteng YANG. Structure-activity relationships of fast-growing wood based hard carbon anodes for sodium ion battery [J]. Energy Storage Science and Technology, 2025, 14(3): 1107-1114. |
| [7] | Shuaibo ZENG, Yongyi LI, Jing PENG, Zixing HE, Zhuojian LIANG, Wei XU, Lingxiao LAN, Xinghua LIANG. Optimization design of conductive agent based on ternary lithium-ion battery [J]. Energy Storage Science and Technology, 2025, 14(3): 1187-1197. |
| [8] | Yi LIANG, Tao WEI, Guangda YIN, Dequan HUANG. Design of a lithiophilic Ag-3D-Cu electrode and its electrochemical performance [J]. Energy Storage Science and Technology, 2025, 14(2): 515-524. |
| [9] | Lishuai ZHANG, Yifei ZHANG, Yiyang MA, Sibo ZHAO, Hongquan LIU, Shengting SHI, Yanjun ZHONG. Research progress on sodium-ion battery cathode materials based on iron-based prussian blue analogues [J]. Energy Storage Science and Technology, 2025, 14(2): 525-543. |
| [10] | Xunchang JIANG, Kelin YU, Daxiang YANG, Minhui LIAO, Yang ZHOU. Preparation of PDOL-based solid electrolyte by in-situ polymerization and its application in lithium metal batteries [J]. Energy Storage Science and Technology, 2025, 14(1): 1-12. |
| [11] | Yinan HE, Kai ZHANG, Junwu ZHOU, Xinyang WANG, Bailin ZHENG. Influence of external loads on the cycling performance of silicon anode lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(8): 2559-2569. |
| [12] | Yinbao MIAO, Wenhua ZHANG, Weihao LIU, Shuai WANG, Zhe CHEN, Wang PENG, Jie ZENG. Preparation and performance of lithium-rich cathode material Li1.2Ni0.13Co0.13Mn0.54O2 [J]. Energy Storage Science and Technology, 2024, 13(5): 1427-1434. |
| [13] | Xin LIU, Xiling MAO, Xinyu YAN, Junqiang WANG, Mengwei LI. Preparation and electrochemical properties of NiMn-MOF with 3D pore network electrode materials [J]. Energy Storage Science and Technology, 2024, 13(2): 361-369. |
| [14] | Yang ZHOU, Peiyu HAN, Yingchun NIU, Chunming XU, Quan XU. Fabrication of metal-organic framework-derived C-Bi/CC electrode materials and their electrochemical properties in ICRFB [J]. Energy Storage Science and Technology, 2024, 13(2): 381-389. |
| [15] | Shun LI, Jianguo HUANG, Guijin HE. Lignin-based carbon/sulfur nanosphere composite as a cathode material for high-performance lithium-sulfur batteries [J]. Energy Storage Science and Technology, 2024, 13(1): 270-278. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
