Energy Storage Science and Technology ›› 2024, Vol. 13 ›› Issue (6): 1849-1860.doi: 10.19799/j.cnki.2095-4239.2023.0929
• Energy Storage Materials and Devices • Previous Articles Next Articles
Ran XU1( ), Baodong WANG2, Shaoliang WANG1, Qi ZHANG1, Lei ZHANG1, Ziyang FENG1
), Baodong WANG2, Shaoliang WANG1, Qi ZHANG1, Lei ZHANG1, Ziyang FENG1
Received:2023-12-22
Revised:2023-12-27
Online:2024-06-28
Published:2024-06-26
Contact:
Ran XU
E-mail:20063985@chnenergy.com.cn
CLC Number:
Ran XU, Baodong WANG, Shaoliang WANG, Qi ZHANG, Lei ZHANG, Ziyang FENG. Research progress on heteroatom-doped electrodes used in all vanadium redox flow batteries[J]. Energy Storage Science and Technology, 2024, 13(6): 1849-1860.
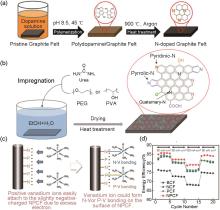
Fig. 2
(a) Schematic diagrams of the dopamine-derived graphite felt fabrication process[19]; (b) Synthesis procedure of the N and O dual-doped GF electrodes by urea thermolysis[25]; (c) Schematic of catalytic mechanism toward the VO2+/VO2+ redox reaction of a N, P co-doped carbon felt carbon framework doped with N and P atoms[26]; (d) Energy efficiency of VRFB cells employing the NPCF at various current densities[26]"
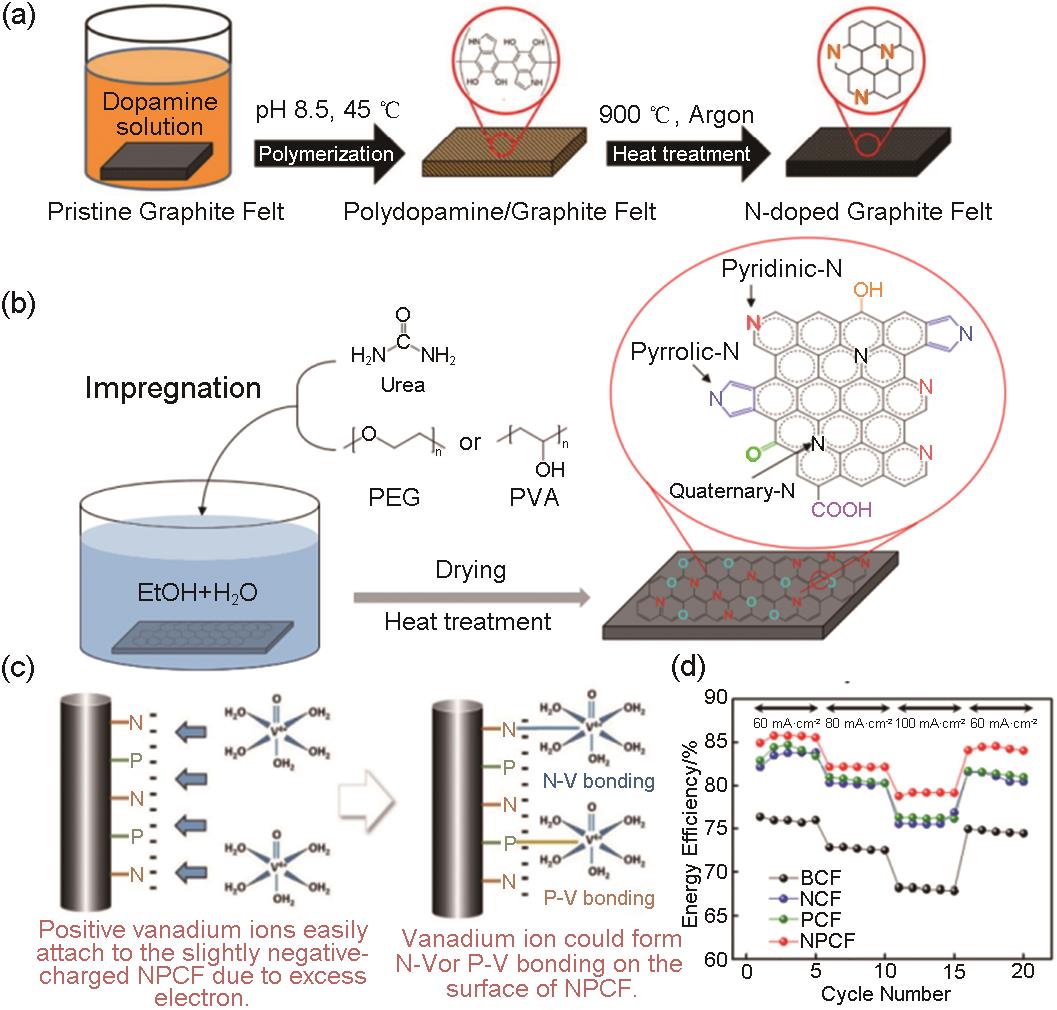
Fig. 3
(a) The energy barriers to remove the boron atom from carbon surfaces. The brown and green balls represent the carbon and boron atoms, respectively; (b) Electrochemical impedance spectroscopy analysis results of CFFB samples[28]; (c) Cyclic votammograms on different electrodes at a scan rate of 30 mV/s[32]; (d) SEM images of B-CNF-N[31]; (e) the synthesis procedure of the nitrogen, boron, and oxygen doped GF electrodes[16]; (f) Energy efficiency of VRFB cells employing the different GF electrodes at various current densities[16]"
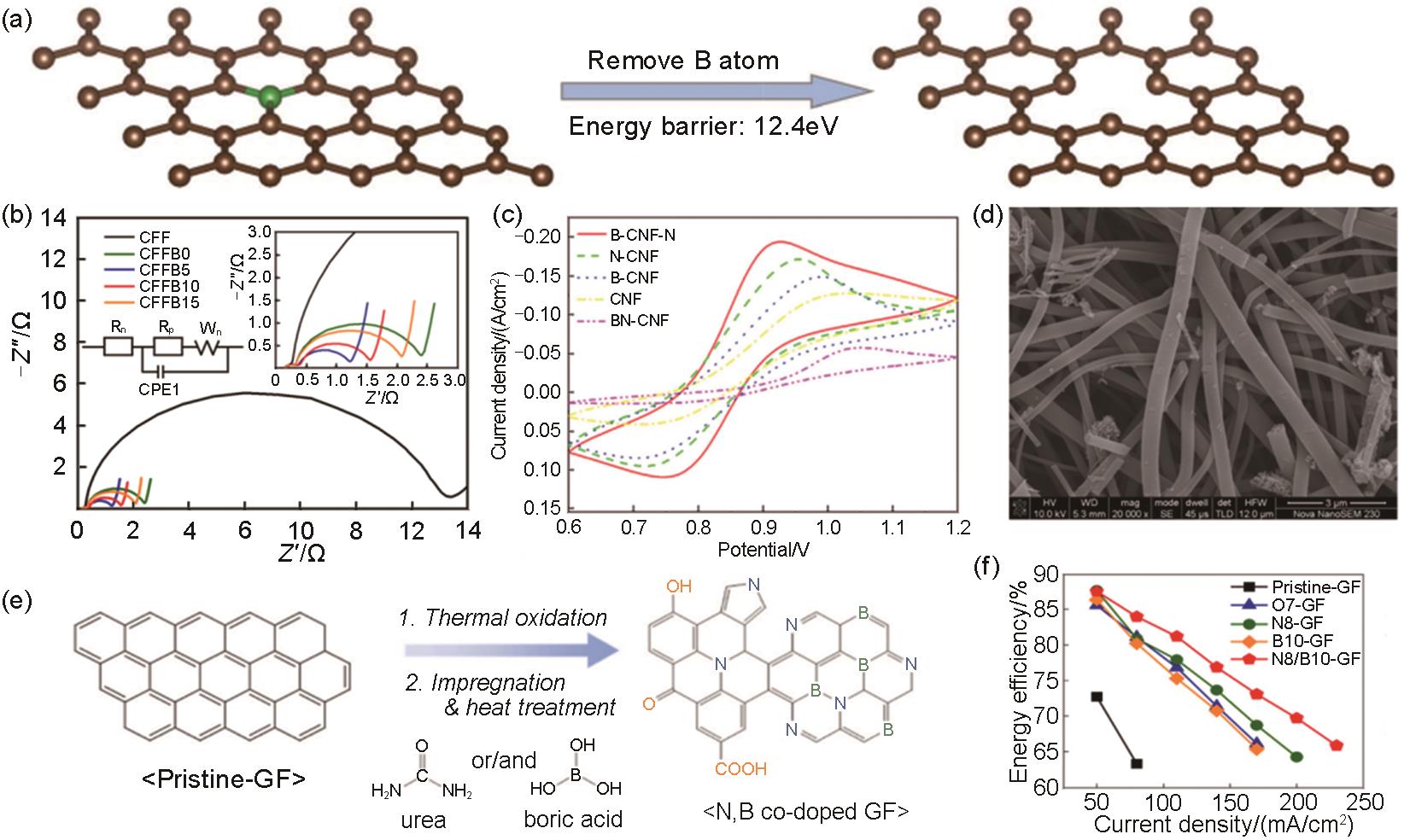

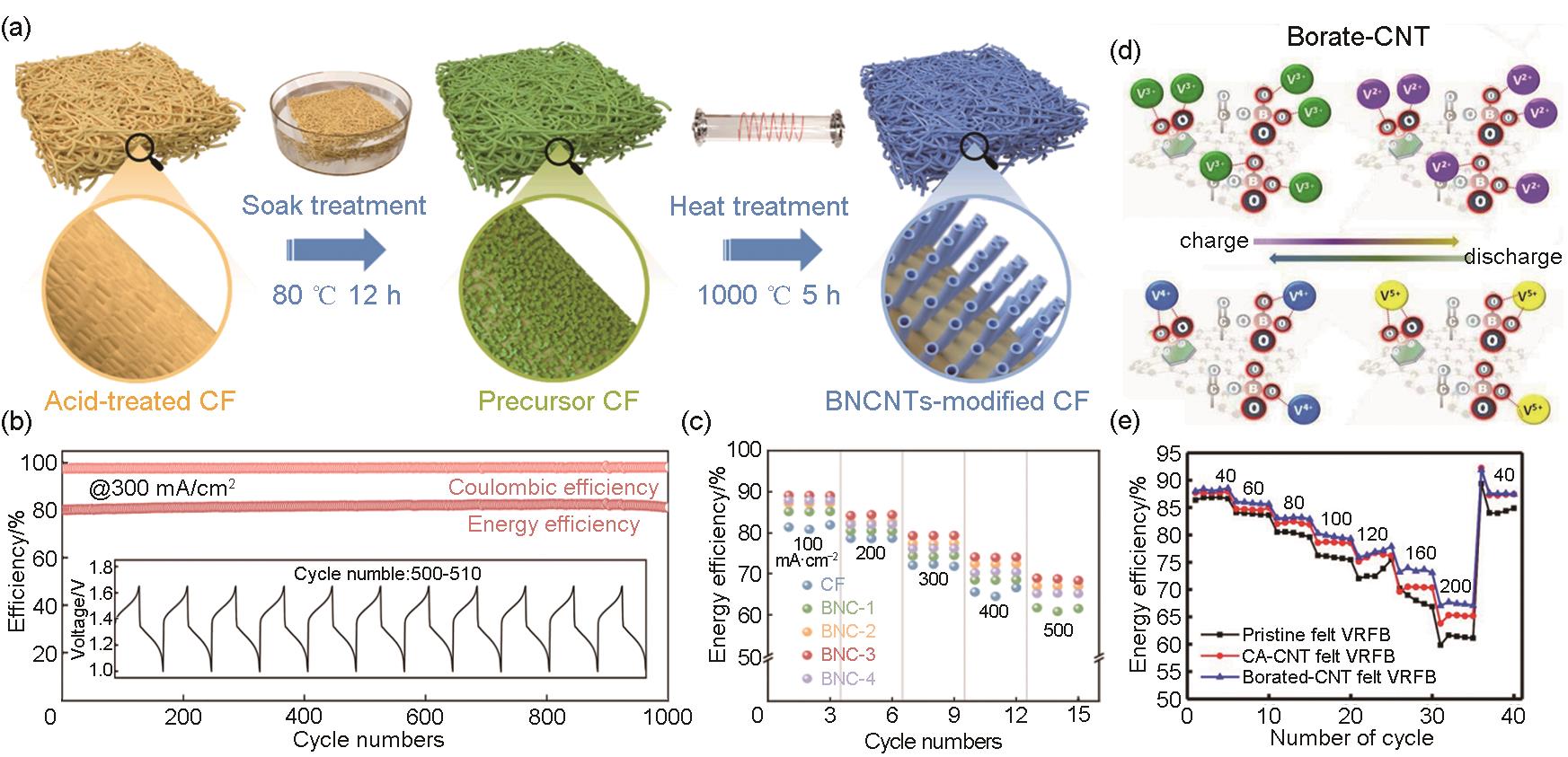
Fig. 5
(a) Diagram illustrating the in-situ method for synthesizing BNC[40]; (b) Long-cycle performance[40]; (c) Energy efficiency of VRFB cells employing the BNC[40]; (d) The suggested redox reaction mechanisms of vanadium ions with BA-CNT catalys[13]; (e) energy efficiency of VRFBs using BA-CNT coated felt for both electrodes[13]"
| 1 | 贾传坤, 王庆. 高能量密度液流电池的研究进展[J]. 储能科学与技术,2015, 4(5): 467-475. |
| JIA C K, WANG Q.The development of high energy density redox flow batteries[J].Energy Storage Science and Technology, 2015, 4(5): 467-475. | |
| 2 | XUAN T, WANG L W. Eutectic electrolyte and interface engineering for redox flow batteries[J]. Energy Storage Materials, 2022, 48: 263-282. |
| 3 | GENCTEN M, SAHIN Y. A critical review on progress of the electrode materials of vanadium redox flow battery[J]. Energy Research, 2020, 44: 7903-7923. |
| 4 | KUMAR R, SAHOO S, JOANNI E, et al. Heteroatom doped graphene engineering for energy storage and conversion[J]. Materials Today, 2020, 39: 47-65. |
| 5 | 李强, 王俊楠, 孙红, 等. 钒液流电池石墨毡电极的MWCNTs-COOH-NS修饰[J]. 储能科学与技术, 2021, 10(6): 2097-2105. |
| LI Q, WANG J N, SUN H, et al. Graphite felt electrode modified with MWCNTs-COOH-NS for vanadium flow battery[J]. Energy Storage Science and Technology, 2021, 10(6): 2097-2105. | |
| 6 | 姚祯, 张琦, 王锐, 等. 生物质衍生碳材料在全钒液流电池电极方面的应用[J]. 储能科学与技术, 2022, 11(7): 2083-2091. |
| YAO Z, ZHANG Q, WANG R, et al. Application of biomass derived carbon materials in all vanadium flow battery electrodes[J]. Energy Storage Science and Technology, 2022, 11(7): 2083-2091. | |
| 7 | 曾艳, 吕早生, 刘俞辰, 等. 全钒液流电池中石墨毡电极的改性研究[J]. 电镀与精饰, 2019, 41(1): 15-21. |
| ZENG Y, LV Z S, LIU Y C, et al. Recent progress on graphite felt as electrode materials in vanadium redox flow battery[J]. Plating and Finishing, 2019, 41(1): 15-21. | |
| 8 | SANKARALINGAM R K, SESHADRI S, SUNARSO J, et al. Overview of the factors affecting the performance of vanadium redox flow batteries[J]. Journal of Energy Storage, 2021, 41, 102857. |
| 9 | 娄景媛, 尤东江, 李雪菁, 等. 全钒氧化还原液流电池用石墨毡电极的分步氧化活化[J]. 电化学, 2020, 26(6): 876-884. |
| LOU J Y, YOU L J, LI X J, et al. Step-by-step modification of graphite felt electrode for vanadium redox flow battery[J]. Journal of electrochemistry, 2020, 26(6): 876-884. | |
| 10 | QI S T, DAI L, HUO W J, et al. Doping engineering strategies for electrodes and catalysts in vanadium redox flow battery[J]. Composites Part B, 2023, doi: 10.1016/j.compositesb.2023.110947. |
| 11 | LIU JING, SONG P, NING Z G, et al. Recent advances in heteroatom-doped metal-free electrocatalysts for highly efficient oxygen reduction reaction[J]. Electrocatalysis, 2015, 6: 132-147. |
| 12 | ZHANG K Y, YAN C W, TANG A. Interfacial co-polymerization derived nitrogendoped carbon enables high-performance carbon felt for vanadium flow batteries[J]. Journal of Materials Chemistry A, 2021, 9, 17300-17310. |
| 13 | HE Z X, JIANG Y Q, ZHU J, et al., Phosphorus doped multi-walled carbon nanotubes: An excellent electrocatalyst for the VO2+/VO2 + redox Reaction[J]. ChemElectroChem, 2018, 5(17): 2464-2474. |
| 14 | CHUNG Y, NOH C, KWON Y. Role of borate functionalized carbon nanotube catalyst for the performance improvement of vanadium redox flow battery[J]. Journal of Power Sources, 2019, 438, 227063. |
| 15 | 苏秀丽, 杨霖霖, 周禹, 等, 全钒液流电池电极研究进展[J]. 储能科学与技术, 2019, 8(1): 65-74. |
| SU X L, YANG L L, ZHOU Y, et al. Developments of electrodes for vanadium redox flow battery[J]. Energy Storage Science and Technology, 2019, 8(1): 65-74. | |
| 16 | KIM H, PAICK J, YI J S, et al. Kinetic relevancy of surface defects and heteroatom functionalities of carbon electrodes for the vanadium redox reactions in flow batteries[J]. Journal of Power Sources, 2023, 557: 232612. |
| 17 | JIN J T, FU X G, LIU Y R, et al. Identifying the active site in nitrogen-doped graphene for the VO2+/VO2 + redox reaction[J]. ACS Nano, 2013, 7(6): 4764-4773 |
| 18 | WU L T, SHEN Y, YU L H, et al. Boosting vanadium flow battery performance by nitrogen-doped carbon nanospheres electrocatalyst[J]. Nano Energy, 2016, 28: 19-28. |
| 19 | LEE H J, KIM H. Graphite felt coated with dopamine-derived nitrogen-doped carbon as a positive electrode for a vanadium redox flow battery[J]. Journal of The Electrochemical Society, 2015, 162(8): A1675-A1681. |
| 20 | HE Z Q, ZHOU X J, ZHANG Y, et al. Low-temperature nitrogen-doping of graphite felt electrode for vanadium redox flow batteries[J]. Journal of The Electrochemical Society, 2019, 166(12): A2336-A2340. |
| 21 | KIM K J, LEE H S, KIM J, et al. Superior electrocatalytic activity of a robust carbon-felt electrode with oxygen-rich phosphate groups for all-vanadium redox flow batteries[J]. ChemSusChem, 2016, 9: 1329-1338. |
| 22 | WANG R, LI Y S, WANG Y N, et al. Phosphorus-doped graphite felt allowing stabilized electrochemical interface and hierarchical pore structure for redox flow battery[J]. Applied Energy, 2020, 261, 114369. |
| 23 | WU X W, XIE Z Y, ZHOU H K, et al. Designing high efficiency graphite felt electrode via HNO3 vapor activation towards stable vanadium redox flow battery[J]. Electrochimica Acta 2023, 440, 141728. |
| 24 | HUANG Y Q, DENG Q, WU X W, et al. N, O co-doped carbon felt for high-performance all-vanadium redox flow battery[J]. International Journal of Hydrogen Energy, 2017, 42: 7177-7185. |
| 25 | KIM S, LIM H, KIM H, et al. Nitrogen and oxygen dual-doping on carbon electrodes by urea thermolysis and its electrocatalytic significance for vanadium redox flow battery[J]. Electrochimica Acta, 2020, 348: 136286. |
| 26 | PARK S E, LEE K, SUHARTO Y, et al. Enhanced electrocatalytic performance of nitrogen-and phosphorous-functionalized carbon felt electrode for VO2+/VO2 + redox reaction[J]. International Journal of Energy Research, 2021, 45: 1806-1817. |
| 27 | OPAR D O, NANKYA R, RAJ C J, et al. In-situ functionalization of binder-free three-dimensional boron-doped mesoporous graphene electrocatalyst as a high-performance electrode material for all-vanadium redox flow batteries[J]. Applied Materials Today, 2021, 22: 100950. |
| 28 | JIANG H R, SHYY W, ZENG L, et al. Highly efficient and ultra-stable boron-doped graphite felt electrodes for vanadium redox flow batteries[J]. Journal of Materials Chemistry A, 2018, 6: 13244-13253. |
| 29 | LEE S, CHO S Y, CHUNG Y S, et al., High electrical and thermal conductivities of a PAN-based carbon fiber via boron-assisted catalytic graphitization[J]. Carbon, 2022, 199: 70-79. |
| 30 | YANG I, LEE S, JANG D, et al. Enhancing energy efficiency and long-term durability of vanadium redox flow battery with catalytically graphitized carbon fiber felts as electrodes by boron doping[J]. Electrochimica Acta, 2022, 429: 141033. |
| 31 | ZHANG J L, LIU Y Y, LU S F, et al. Nitrogen, phosphorus co-doped graphite felt as highly efficient electrode for VO2+/VO2 + reaction[J]. Batteries, 2023, 9(40): 1-10. |
| 32 | SHI L, LIU S Q, HE Z, et al. Synthesis of boron and nitrogen co-doped carbon nanofiber as efficient metal-free electrocatalyst for the VO2+/VO2 + redox reaction[J]. Electrochimica Acta, 2015, 178: 748-757. |
| 33 | ZHANG F J, ZHANG H T,et al. Applications of nanocarbons in redox flow batteries[J]. New Carbon Materials, 2021, 36(1): 82-92. |
| 34 | HUANG R J, WANG Y, LIU S Q. Non-precious transition metal based electrocatalysts for vanadium redox flow batteries: Rational design and perspectives[J]. Journal of Power Sources, 2021, 515: 230640. |
| 35 | XU J, ZHANG Y Q, HUANG Z F, et al. Surface modification ocarbon-based electrodes for vanadium redox flow batteries[J].Energy Fuels, 2021, 35: 8617-8633. |
| 36 | PARK M J, RYU J, CHO J, et al. Nanostructured electrocatalysts for all-vanadium redox flow batteries[J].Chemistry an Asian Journal, 2015, 10: 2096-2110. |
| 37 | LIU Y C, SHEN Y, YU L H, et al. Holey-engineered electrodes for advanced vanadium flow batteries[J]. Nano Energy, 2018, 43: 55-62. |
| 38 | LIU Y B, YU L H, LIU X, et al. ZIF-derived holey electrode with enhanced mass transfer and N-rich catalytic sites for high-power and long-life vanadium flow batteries[J]. Journal of Energy Chemistry, 2022, 72: 545-553. |
| 39 | JI J, NOH C, SHIN M, et al. Vanadium redox flow batteries using new mesoporous nitrogen-doped carbon coated graphite felt electrode[J]. Applied Surface Science, 2023, 611: 155665. |
| 40 | WANG S Y, ZHAO X S, COCHELL T, et al. Nitrogen-doped carbon nanotube/graphite felts as advanced electrode materials for vanadium redox flow batteries[J]. The Journal of Physical Chemistry Letters, 2012, 3: 2164-2167. |
| 41 | LIU L S, ZHANG X H, ZHANG D H, et al. Regulating the N/B ratio to construct B, N co-doped carbon nanotubes on carbon felt for high-performance vanadium redox flow batteries[J]. Chemical Engineering Journal, 2023, 473: 145454. |
| 42 | GONZÁLEZ Z, BOTAS C, BLANCO C, et al. Thermally reduced graphite and graphene oxides in VRFBs[J]. Nano energy, 2013, 2(6): 1322-1328. |
| 43 | LING W, WANG Z A, MA Q, et al. Phosphorus and oxygen co-doped composite electrode with hierarchical electronic and ionic mixed conducting networks for vanadium redox flow batteries[J].Chemical Communications, 2019: 1-4. |
| 44 | LI Q, WANG J N, ZHANG T Y, et al. Analysis of the performance of phosphorus and sulphur co-doped reduced graphene oxide as catalyst in vanadium redox flow battery[J]. Journal of Electrochemical Society, 2022, 169: 010523. |
| 45 | LI Q, LIU J Q, BAI A Y, et al. Preparation of a nitrogen-doped reduced graphene oxide-modified graphite felt electrode for VO2+/VO2 + reaction by freeze-drying and pyrolysis method[J]. Journal of Chemistry, 2019: 8958946. |
| 46 | XU A, SHI L, ZENG L, et al. First-principle investigations of nitrogen, boron, phosphorus-doped graphite electrodes for vanadium redox flow batteries[J]. Electrochimica Acta, 2019, 300: 389-395. |
| 47 | SHI L, LIU S Q, HE Z, et al. Nitrogen-doped graphene:Effects of nitrogen species on the properties of the vanadium redox flow battery[J]. Electrochimica Acta, 2014, 138: 93-100. |
| [1] | Deshuai LIU, Huiqin ZHU, Ruihao SUN, Meng LI, Wenhao GONG, Xiaohui LI, Weiwei QIAN. Synergistic dual-additive boost cyclability of sodium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(5): 1858-1865. |
| [2] | Guipei XU, Hao LIU, Jiewen LAI, Yifeng LU, Hui HUANG, Huifang DI, Zhenbing WANG. Research progress on solvent-free electrode technology for supercapacitor and lithium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(4): 1445-1460. |
| [3] | Zixin XIAO, Hong ZHANG, Lin XU. Nanowires modulating ion transport and interfaces in solid-state lithium batteries [J]. Energy Storage Science and Technology, 2025, 14(3): 1026-1039. |
| [4] | Yuelin LI, Zhiyu LIU, Sen GUO, Xiaojun LIU, Pengliang ZHANG, Chengcheng WANG, Yuan LIANG, Rui WANG. Research progress on electrode structure design of vanadium redox flow battery [J]. Energy Storage Science and Technology, 2025, 14(2): 601-612. |
| [5] | Hong WANG, Kaiyue ZHANG. Study on thermal treatment activation of carbon felt electrode for all-vanadium flow batteries [J]. Energy Storage Science and Technology, 2025, 14(2): 488-496. |
| [6] | Tong LIU, Guiting YANG, Hui BI, Yueni MEI, Shuo LIU, Yongji GONG, Wenlei LUO. Recent progress in high-energy and high-power lithium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(1): 54-76. |
| [7] | Yijie YAO, Junwei ZHANG, Yanjun ZHAO, Hongcheng LIANG, Dongni ZHAO. Effect of interfacial dynamics on low temperature performance of sodium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(1): 30-41. |
| [8] | Kaiyue YANG, Xinbing XIE, Xiaozhong DU. Exploration of lithium battery electrode calendering process based on discrete element method [J]. Energy Storage Science and Technology, 2024, 13(8): 2570-2579. |
| [9] | Yinan HE, Kai ZHANG, Junwu ZHOU, Xinyang WANG, Bailin ZHENG. Influence of external loads on the cycling performance of silicon anode lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(8): 2559-2569. |
| [10] | Sen JIANG, Long CHEN, Chuangchao SUN, Jinze WANG, Ruhong LI, Xiulin FAN. Low-temperature lithium battery electrolytes: Progress and perspectives [J]. Energy Storage Science and Technology, 2024, 13(7): 2270-2285. |
| [11] | Haotian WANG, Yonggang WANG, Xiaoli DONG. Advances in low-temperature organic batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2259-2269. |
| [12] | Zeheng LI, Lei XU, Yuxing YAO, Chong YAN, Ximin ZHAI, Xuechun HAO, Aibing CHEN, Jiaqi HUANG, Xiaofei BIE, Huanli SUN, Lizhen FAN, Qiang ZHANG. A review of electrolyte reducing lithium plating in low-temperature lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2192-2205. |
| [13] | Shuping WANG, Xiankun YANG, Changhao LI, Ziqi ZENG, Yifeng CHENG, Jia XIE. Diethyl ethylphosphonate-based flame-retardant wide-temperature-range electrolyte in lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2161-2170. |
| [14] | Jianhang YANG, Wenting FENG, Junwei HAN, Xinru WEI, Chenyu MA, Changming MAO, Linjie ZHI, Debin KONG. Recent advances in rechargeable Li/Na-Cl2 batteries: From material construction to performance evaluation [J]. Energy Storage Science and Technology, 2024, 13(6): 1824-1834. |
| [15] | Cong SUO, Yangfeng WANG, Zichen ZHU, Yan YANG. Research progress of soft carbon as negative electrodes in sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(6): 1807-1823. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
