Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (2): 583-600.doi: 10.19799/j.cnki.2095-4239.2024.0771
• Energy Storage Materials and Devices • Previous Articles Next Articles
Zhenfei LIANG1( ), Xingxing WANG2, Haochen HU3, Yanhong LI2, Boxue OUYANG2, Xiaoyun SUN3, Ruimao GAO2, Jun YE2, Deren WANG3(
), Xingxing WANG2, Haochen HU3, Yanhong LI2, Boxue OUYANG2, Xiaoyun SUN3, Ruimao GAO2, Jun YE2, Deren WANG3( )
)
Received:2024-08-15
Revised:2024-10-28
Online:2025-02-28
Published:2025-03-18
Contact:
Deren WANG
E-mail:974987025@qq.com;dr_wang@ustb.edu.cn
CLC Number:
Zhenfei LIANG, Xingxing WANG, Haochen HU, Yanhong LI, Boxue OUYANG, Xiaoyun SUN, Ruimao GAO, Jun YE, Deren WANG. Advancements in electrolyte and membrane technologies for zinc-bromine flow batteries[J]. Energy Storage Science and Technology, 2025, 14(2): 583-600.

Fig. 2
(a) Quaternized polysulfone (QNPSU) reaction formation principle[19]; (b) porous polyolefin/polyethylene glycol (PEG) composite membrane working principle diagram[20]; (c) DFT analysis diagram[20]; (d) Zn2+ concentration distribution and electric field distribution simulation diagram[20]; (e) analysis of PEG-passivated zinc ions calculated by DFT[20]; (f) thermal conductivity analysis of PES-BNNs coated composite diaphragm[21]; (g) electrode zinc dendrite growth diagram after the application of PES-BNNs coated composite diaphragm[21]; (h) glass fiber paper composite diaphragm[22]; (i) typical and novel electroactive titanium mesh anode structure[23]"


Fig. 3
(a) Nafion filled porous membrane preparation flow chart[24]; (b) Nafion filled porous membrane amplification diagram[24]; (c) membrane water cluster amplification diagram[25]; (d) ion conductivity curve of membrane after prehydration treatment[25]; (e) bromine adsorption curve of membrane after prehydration treatment[25]; (f) schematic diagram of the interaction between Nafion and Am-SiO2 in Nafion/Am-SiO2 membranes[26]; (g) UV spectra of solutions filtered by different membranes[26]; (h) changes of Br2 diffusers of different membranes over time[26]"

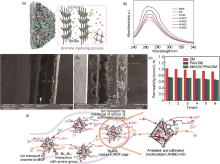
Fig. 5
(a) Schematic diagram of composite membranes[28]; (b) ultraviolet spectra of bromine solutions containing different membranes[28]; (c) surface and cross-sectional images of Daramic membrane[29]; (d) surface and cross-sectional images of MWCNT/PAN-Daramic membrane[29]; (e) the bromine permeability of the prepared membranes[29]; (f) schematic diagram of mechanisms of ion transport and Br2 capture by the U-AS modified membraneduring the ZBFLBs operation[30]"
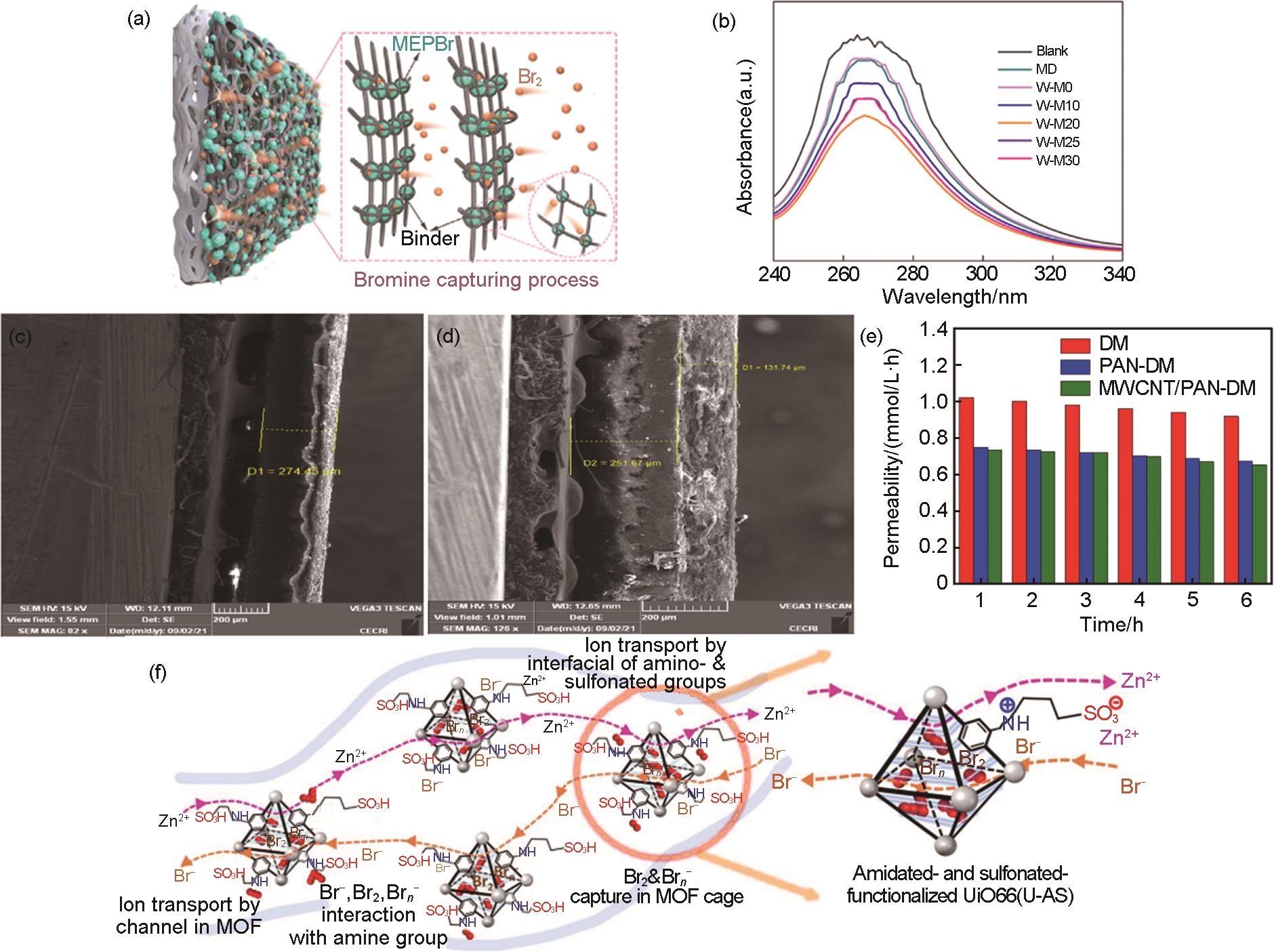

Fig. 6
(a) Schematic diagram of 3D honeycomb lattice as a ZBFBs cell structure[31], (b) schematic diagram of A1C n channel configuration, where n increases from 1 to 4[31], (c) 4-channel (2×2), 8-channel (2×4), and 16-channel (4×4) cell diagram of HC-ZBFBs[31], (d) schematic illustration of the function of the MesoTi3C2-wrapped PP separator in eliminating Zn0 dendrites and the underlying mechanisms[32], (e) the spontaneous redox process between Zn0 and the Ti-Oe functional group on MesoTi3C2[32]"


Fig. 9
(a) Zinc dendrite image with 1 mol/L MSA inserted into the edge of graphite felt[43]; (b) local carbon fiber image with 1 mol/L MSA inserted into the edge of graphite felt[43]; (c) zinc dendrite image without MSA inserted into the edge of graphite felt[43]; (d) local carbon fiber image without MSA inserted into the edge of graphite felt[43]"
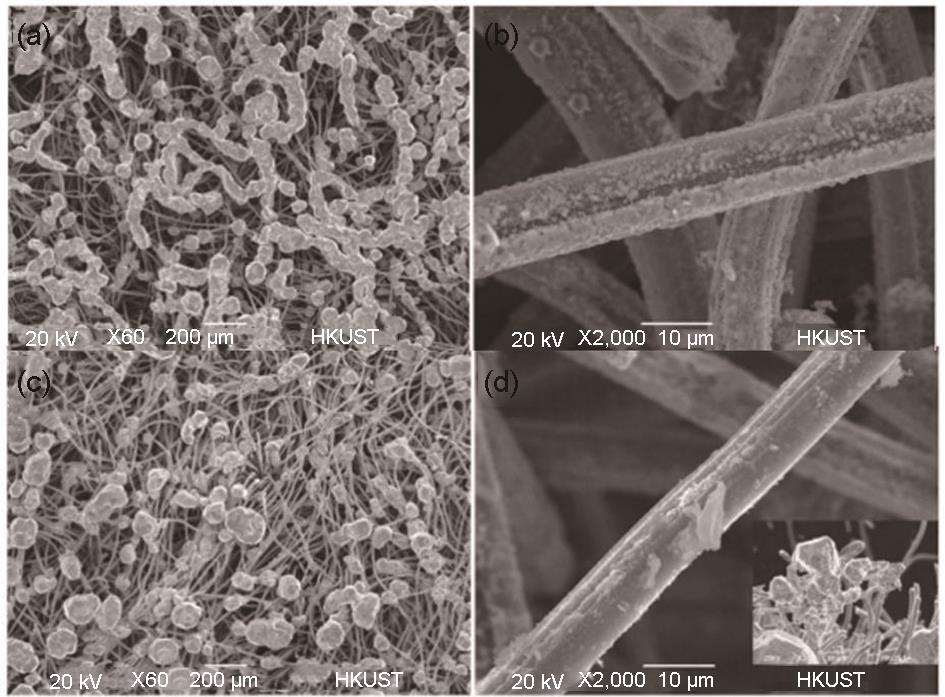

Fig. 11
(a) After 25 charge and discharge cycles, SEM image of carbon slipper (original, supported zinc chloride, supported zinc perchlorate) of a 9 cm2 microbattery[45]; (b) schematic diagram of the migration process of Cl atoms at the Zn site calculated by DFT[47]; (c) enlarged view of zinc deposition under an optical microscope with a PEG electrode[49]; (d) schematic diagram of step-by-step zinc reduction and deposition process under negative potential bias of zinc electrode and PEG-200 electrolyte[35]; (e)schematic diagram of electrostatic shielding effect[50]; (f) schematic diagram of Cr3+ additive explaining Cr3+ ion shielding of zinc seed[36]"

Fig. 15
(a) At a current density of 20 mA/cm2, The distribution of bromine concentration; (b) in the positive electrode at the end of battery discharge at different flow rates of positive electrolyte is 20 mA/cm2 and 40 Current, Coulomb, and energy efficiency of batteries with different positive electrolyte flow rates at mA/cm2; (c) energy efficiency of batteries with different electrolyte flow rates and different electrode thicknesses; (d) energy efficiency of batteries with different electrolyte flow rates and different electrode porosity[65]"

| 1 | ANG T Z, SALEM M, KAMAROL M, et al. A comprehensive study of renewable energy sources: Classifications, challenges and suggestions[J]. Energy Strategy Reviews, 2022, 43: 100939. DOI:10.1016/j.esr.2022.100939. |
| 2 | YANG Z G, ZHANG J L, KINTNER-MEYER M C W, et al. Electrochemical energy storage for green grid[J]. Chemical Reviews, 2011, 111(5): 3577-3613. DOI:10.1021/cr100290v. |
| 3 | YANG Z G, LIU J, BASKARAN S, et al. Enabling renewable energy—And the future grid—With advanced electricity storage[J]. JOM, 2010, 62(9): 14-23. DOI:10.1007/s11837-010-0129-0. |
| 4 | DEGUENON L, YAMEGUEU D, MOUSSA KADRI S, et al. Overcoming the challenges of integrating variable renewable energy to the grid: A comprehensive review of electrochemical battery storage systems[J]. Journal of Power Sources, 2023, 580: 233343. DOI:10.1016/j.jpowsour.2023.233343. |
| 5 | YANG Y Q, BREMNER S, MENICTAS C, et al. Battery energy storage system size determination in renewable energy systems: A review[J]. Renewable and Sustainable Energy Reviews, 2018, 91: 109-125. DOI:10.1016/j.rser.2018.03.047. |
| 6 | MENÉNDEZ J, FERNÁNDEZ-ORO J M, GALDO M, et al. Efficiency analysis of underground pumped storage hydropower plants[J]. Journal of Energy Storage, 2020, 28: 101234. DOI:10.1016/j.est.2020.101234. |
| 7 | ZHANG L Y, YU G H. Recent developments in materials and chemistries for redox flow batteries[J]. ACS Materials Letters, 2023, 5(11): 3007-3009. DOI:10.1021/acsmaterialslett.3c01191. |
| 8 | LIM H S, LACKNER A M, KNECHTLI R C. Zinc-bromine secondary battery[J]. Journal of the Electrochemical Society, 124(8): 1154-1157. DOI:10.1149/1.2133517. |
| 9 | WANG W, LUO Q T, LI B, et al. Recent progress in redox flow battery research and development[J]. Advanced Functional Materials, 2013, 23(8): 970-986. DOI:10.1002/adfm.201200694. |
| 10 | NOACK D J, ROZNYATOVSKAYA D N, HERR T, et al. The chemistry of redox-flow batteries[J]. Angewandte Chemie International Edition, 2015, 54(34): 9776-9809. DOI:10.1002/anie.201410823. |
| 11 | XU P C, LI T Y, ZHENG Q, et al. A low-cost bromine-fixed additive enables a high capacity retention zinc-bromine batteries[J]. Journal of Energy Chemistry, 2022, 65: 89-93. DOI:10.1016/j.jechem.2021.05.036. |
| 12 | KIM M, YUN D, JEON J. Effect of a bromine complex agent on electrochemical performances of zinc electrodeposition and electrodissolution in Zinc-Bromide flow battery[J]. Journal of Power Sources, 2019, 438: 227020. DOI:10.1016/j.jpowsour. 2019.227020. |
| 13 | 刘金宇, 李丹, 王丽华, 等. 高质子选择性的SPEEK/SGO质子交换膜制备及钒电池应用[J]. 储能科学与技术, 2018, 7(1): 66-74. DOI: 10.12028/j.issn.2095-4239.2017.0148. |
| LIU J Y, LI D, WANG L H, et al. SPEEK/SGO proton exchange membranes with superior proton selectivity for vanadium redox battery[J]. Energy Storage Science and Technology, 2018, 7(1): 66-74. DOI: 10.12028/j.issn.2095-4239.2017.0148. | |
| 14 | 王茜. 筛分效应Nafion复合膜及其全钒液流电池性能研究[D]. 大连:大连理工大学. |
| WANG Q. Study on the performance of Nafion composite membrane and all-vanadium flow battery with screening effect [D]. Dalian: Dalian University of Technology, 2018. | |
| 15 | 张祺, 张苗苗, 孟琳. N-甲基-N-丁基吡咯烷溴化物和N-甲基-N-乙基吡咯烷溴化物在锌溴液流电池中的应用[J]. 电化学, 2017, 23(6): 694-701. |
| ZHANG Q, ZHANG M M, MENG L. Applications of N-methyl-N-butyl-pyrrolidinium bromide and N-methyl-N-ethyl-pyrrolidinium bromide in Zn-Br flow batteries[J]. Journal of Electrochemistry, 2017, 23(6): 694-701. | |
| 16 | HUANG Z B, MU A L, WU L X, et al. Comprehensive analysis of critical issues in all-vanadium redox flow battery[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(24): 7786-7810. DOI:10.1021/acssuschemeng.2c01372. |
| 17 | DAI C L, HU L Y, JIN X T, et al. Fast constructing polarity-switchable zinc-bromine microbatteries with high areal energy density[J]. Science Advances, 2022, 8(28): eabo6688. DOI:10.1126/sciadv.abo6688. |
| 18 | BISWAS S, SENJU A, MOHR R, et al. Minimal architecture zinc-bromine battery for low cost electrochemical energy storage[J]. Energy & Environmental Science, 2017, 10(1): 114-120. DOI:10.1039/C6EE02782B. |
| 19 | LI M Q, SU H, QIU Q G, et al. A quaternized polysulfone membrane for zinc-bromine redox flow battery[J]. Journal of Chemistry, 2014, 2014(1): 321629. DOI:10.1155/2014/321629. |
| 20 | LU W J, LI T Y, YUAN C G, et al. Advanced porous composite membrane with ability to regulate zinc deposition enables dendrite-free and high-areal capacity zinc-based flow battery[J]. Energy Storage Materials, 2022, 47: 415-423. DOI:10.1016/j.ensm.2022.02.034. |
| 21 | HU J, YUE M, ZHANG P H, et al. A boron nitride nanosheets composite membrane for a long-life zinc-based flow battery[J]. Angewandte Chemie International Edition, 2020, 59(17): 6715-6719. DOI:10.1002/anie.201914819. |
| 22 | KIM R, JUNG J, LEE J H, et al. Modulated Zn deposition by glass fiber interlayers for enhanced cycling stability of Zn-Br redox flow batteries[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(36): 12242-12251. DOI:10.1021/acssuschemeng.1c03796. |
| 23 | LEE J N, DO E, KIM Y, et al. Development of titanium 3D mesh interlayer for enhancing the electrochemical performance of zinc-bromine flow battery[J]. Scientific Reports, 2021, 11(1): 4508. DOI:10.1038/s41598-021-83347-1. |
| 24 | KIM R, KIM H G, DOO G, et al. Ultrathin Nafion-filled porous membrane for zinc/bromine redox flow batteries[J]. Scientific Reports, 2017, 7(1): 10503. DOI:10.1038/s41598-017-10850-9. |
| 25 | KIM R, YUK S, LEE J H, et al. Scaling the water cluster size of Nafion membranes for a high performance Zn/Br redox flow battery[J]. Journal of Membrane Science, 2018, 564: 852-858. DOI:10.1016/j.memsci.2018.07.091. |
| 26 | HAN D B, SHANMUGAM S. Active material crossover suppression with bi-ionic transportability by an amphoteric membrane for Zinc-Bromine redox flow battery[J]. Journal of Power Sources, 2022, 540: 231637. DOI:10.1016/j.jpowsour. 2022.231637. |
| 27 | GIKUNOO E K, HAN D B, VINOTHKANNAN M, et al. Synthesis and characterization of highly durable hydrocarbon-based composite membrane for zinc-bromine redox flow battery[J]. Journal of Power Sources, 2023, 563: 232821. DOI:10.1016/j.jpowsour.2023.232821. |
| 28 | HUA L, LU W, LI T, et al. A highly selective porous composite membrane with bromine capturing ability for a bromine-based flow battery[J]. Materials Today Energy, 2021, 21: 100763. DOI:10.1016/j.mtener.2021.100763. |
| 29 | NARESH R P, RAGUPATHY P, ULAGANATHAN M. Carbon nanotube scaffolds entrapped in a gel matrix for realizing the improved cycle life of zinc bromine redox flow batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(40): 48110-48118. DOI:10.1021/acsami.1c14324. |
| 30 | HAN D B, SHIN K, KIM H T, et al. Functionalized metal-organic framework modified membranes with ultralong cyclability and superior capacity for zinc/bromine flowless batteries[J]. Journal of Materials Chemistry A, 2024, 12(23): 13970-13979. DOI:10.1039/D4TA01005A. |
| 31 | CHA J S, LEE J I, SEO N U, et al. Scalable design of zinc-bromine flow battery in 3-dimensional honeycomb lattice for superior low-cost battery[J]. SSRN Electronic Journal, DOI:10.2139/ssrn.4089539 |
| 32 | BU F X, SUN Z H, ZHOU W H, et al. Reviving Zn0 dendrites to electroactive Zn2+ by mesoporous MXene with active edge sites[J]. Journal of the American Chemical Society, 2023, 145(44):24284-24293. |
| 33 | MAHMOOD A, ZHENG Z, CHEN Y. Zinc-bromine batteries: Challenges, prospective solutions, and future[J]. Advanced Science, 2024, 11(3): 2305561. DOI:10.1002/advs.202305561. |
| 34 | XU Z C, WANG J, YAN S C, et al. Modeling of Zinc Bromine redox flow battery with application to channel design[J]. Journal of Power Sources, 2020, 450: 227436. DOI:10.1016/j.jpowsour. 2019.227436. |
| 35 | MITHA A, YAZDI D A Z, AHMED M, et al. Surface adsorption of polyethylene glycol to suppress dendrite formation on zinc anodes in rechargeable aqueous batteries[J]. ChemElectroChem, 2018, 5(17): 2409-2418. DOI:10.1002/celc.201800572. |
| 36 | BAE S, LEE J, KIM D S. The effect of Cr3+-Functionalized additive in zinc-bromine flow battery[J]. Journal of Power Sources, 2019, 413: 167-173. DOI:10.1016/j.jpowsour.2018.12.038. |
| 37 | YANG J H, YANG H S, RA H W, et al. Effect of a surface active agent on performance of zinc/bromine redox flow batteries: Improvement in current efficiency and system stability[J]. Journal of Power Sources, 2015, 275: 294-297. DOI:10.1016/j.jpowsour. 2014.10.208. |
| 38 | HAN S H, KIM S, LIM H Y, et al. Modified viologen-assisted reversible bromine capture and release in flowless zinc-bromine batteries[J]. Chemical Engineering Journal, 2023, 464: 142624. DOI:10.1016/j.cej.2023.142624. |
| 39 | ZHANG L Q, ZHANG H M, LAI Q Z, et al. Development of carbon coated membrane for zinc/bromine flow battery with high power density[J]. Journal of Power Sources, 2013, 227: 41-47. DOI:10.1016/j.jpowsour.2012.11.033. |
| 40 | MUNAIAH Y, DHEENADAYALAN S, RAGUPATHY P, et al. High performance carbon nanotube based electrodes for zinc bromine redox flow batteries[J]. ECS Journal of Solid State Science and Technology, 2013, 2(10): M3182-M3186. DOI:10.1149/2.024310jss. |
| 41 | WU M C, ZHAO T S, JIANG H R, et al. High-performance zinc bromine flow battery via improved design of electrolyte and electrode[J]. Journal of Power Sources, 2017, 355: 62-68. DOI:10.1016/j.jpowsour.2017.04.058. |
| 42 | KIM D, JEON J. Study on durability and stability of an aqueous electrolyte solution for zinc bromide hybrid flow batteries[J]. Journal of Physics: Conference Series, 2015, 574: 012074. DOI:10.1088/1742-6596/574/1/012074. |
| 43 | WU M C, ZHAO T S, WEI L, et al. Improved electrolyte for zinc-bromine flow batteries[J]. Journal of Power Sources, 2018, 384: 232-239. DOI:10.1016/j.jpowsour.2018.03.006. |
| 44 | WANG S N, WANG Z Y, YIN Y B, et al. A highly reversible zinc deposition for flow batteries regulated by critical concentration induced nucleation[J]. Energy & Environmental Science, 2021, 14(7): 4077-4084. DOI:10.1039/D1EE00783A. |
| 45 | KIM D, JEON J. A Zn(ClO4)2 supporting material for highly reversible zinc-bromine electrolytes[J]. Bulletin of the Korean Chemical Society, 2016, 37(3): 299-304. DOI:10.1002/bkcs.10669. |
| 46 | RAJARATHNAM G P, SCHNEIDER M, SUN X H, et al. The influence of supporting electrolytes on zinc half-cell performance in zinc/bromine flow batteries[J]. Journal of the Electrochemical Society, 2016, 163(1): A5112-A5117. DOI:10.1149/2.0151601jes. |
| 47 | RAJARATHNAM G P, MONTOYA A, VASSALLO A M. The influence of a chloride-based supporting electrolyte on electrodeposited zinc in zinc/bromine flow batteries[J]. Electrochimica Acta, 2018, 292: 903-913. DOI:10.1016/j.electacta.2018.08.150. |
| 48 | SUN K E K, HOANG T K A, DOAN T N L, et al. Suppression of dendrite formation and corrosion on zinc anode of secondary aqueous batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(11): 9681-9687. DOI:10.1021/acsami.6b16560. |
| 49 | BANIK S J, AKOLKAR R. Suppressing dendrite growth during zinc electrodeposition by PEG-200 additive[J]. Journal of the Electrochemical Society, 2013, 160(11): D519-D523. DOI:10.1149/2.040311jes. |
| 50 | LEE Y, YUN D, PARK J, et al. An organic imidazolium derivative additive inducing fast and highly reversible redox reactions in zinc-bromine flow batteries[J]. Journal of Power Sources, 2022, 547: 232007. DOI:10.1016/j.jpowsour.2022.232007. |
| 51 | JEON J D, YANG H S, SHIM J, et al. Dual function of quaternary ammonium in Zn/Br redox flow battery: Capturing the bromine and lowering the charge transfer resistance[J]. Electrochimica Acta, 2014, 127: 397-402. DOI:10.1016/j.electacta.2014.02.073. |
| 52 | WANG S N, LI T Y, YIN Y B, et al. High-energy-density aqueous zinc-based hybrid supercapacitor-battery with uniform zinc deposition achieved by multifunctional decoupled additive[J]. Nano Energy, 2022, 96: 107120. DOI:10.1016/j.nanoen. 2022.107120. |
| 53 | PARK H, PARK G, KUMAR S, et al. Synergistic effect of electrolyte additives on the suppression of dendrite growth in a flowless membraneless Zn-Br2 battery[J]. Journal of Power Sources, 2023, 580: 233212. DOI:10.1016/j.jpowsour.2023.233212. |
| 54 | LIU S Y, WU J, HUANG J Q, et al. A high-energy efficiency static membrane-free zinc-bromine battery enabled by a high concentration hybrid electrolyte[J]. Sustainable Energy & Fuels, 2022, 6(4): 1148-1155. DOI:10.1039/D1SE01749G. |
| 55 | JIMÉNEZ-BLASCO U, ARREBOLA J C, CABALLERO A. Recent advances in bromine complexing agents for zinc-bromine redox flow batteries[J]. Materials, 2023, 16(23): 7482. DOI:10.3390/ma16237482. |
| 56 | LAI Q Z, ZHANG H M, LI X F, et al. A novel single flow zinc-bromine battery with improved energy density[J]. Journal of Power Sources, 2013, 235: 1-4. DOI:10.1016/j.jpowsour. 2013.01.193. |
| 57 | BRYANS D, MCMILLAN B G, SPICER M, et al. Complexing additives to reduce the immiscible phase formed in the hybrid ZnBr2 flow battery[J]. Journal of the Electrochemical Society, 2017, 164(13): A3342-A3348. |
| 58 | XU P C, XIE C X, WANG C H, et al. A membrane-free interfacial battery with high energy density[J]. Chemical Communications, 2018, 54(82): 11626-11629. DOI:10.1039/c8cc06048g. |
| 59 | GAO L J, LI Z X, ZOU Y P, et al. A high-performance aqueous zinc-bromine static battery[J]. iScience, 2020, 23(8): 101348. DOI:10.1016/j.isci.2020.101348. |
| 60 | LI X J, LI T Y, XU P C, et al. A complexing agent to enable a wide-temperature range bromine-based flow battery for stationary energy storage[J]. Advanced Functional Materials, 2021, 31(22): 2100133. DOI:10.1002/adfm.202100133. |
| 61 | WU W L, XU S C, LIN Z R, et al. A polybromide confiner with selective bromide conduction for high performance aqueous zinc-bromine batteries[J]. Energy Storage Materials, 2022, 49: 11-18. DOI:10.1016/j.ensm.2022.03.052. |
| 62 | SCHNEIDER M, RAJARATHNAM G P, EASTON M E, et al. The influence of novel bromine sequestration agents on zinc/bromine flow battery performance[J]. RSC Advances, 2016, 6(112): 110548-110556. DOI:10.1039/C6RA23446A. |
| 63 | YANG H S, PARK J H, RA H W, et al. Critical rate of electrolyte circulation for preventing zinc dendrite formation in a zinc-bromine redox flow battery[J]. Journal of Power Sources, 2016, 325: 446-452. DOI:10.1016/j.jpowsour.2016.06.038. |
| 64 | ADITH R V, NARESH R P, MARIYAPPAN K, et al. An optimistic approach on flow rate and supporting electrolyte for enhancing the performance characteristics of Zn-Br2 redox flow battery[J]. Electrochimica Acta, 2021, 388: 138451. DOI:10.1016/j.electacta. 2021.138451. |
| 65 | ZHANG Y Q, WANG G X, LIU R Y, et al. Operational parameter analysis and performance optimization of zinc-bromine redox flow battery[J]. Energies, 2023, 16(7): 3043. DOI:10.3390/en16073043. |
| 66 | ZHANG H M, LU W J, LI X F. Progress and perspectives of flow battery technologies[J]. Electrochemical Energy Reviews, 2019, 2(3): 492-506. DOI:10.1007/s41918-019-00047-1. |
| [1] | Xueru LI, Zhejie MA, Ping LI. Research progress on microstructure characterization of cathode catalyst layer in proton exchange membrane fuel cells [J]. Energy Storage Science and Technology, 2025, 14(2): 812-821. |
| [2] | Yuchen GAO, Weilin LI, Xiang CHEN, Yuhang YUAN, Yilin NIU, Qiang ZHANG. A perspective on DeepSeek application in energy storage research [J]. Energy Storage Science and Technology, 2025, 14(2): 467-478. |
| [3] | Yukun XU, Jun YU, Chao JIANG, Jinghua WANG, Wanru ZHAO. Cost analysis of energy storage technology and power system optimization design [J]. Energy Storage Science and Technology, 2025, 14(2): 876-878. |
| [4] | Aimin SUN, Jianjun HONG, Jianfeng ZHENG. Analysis of the application of energy storage technology in the renewable energy grid-connected operation [J]. Energy Storage Science and Technology, 2025, 14(2): 879-882. |
| [5] | Tong LIU, Guiting YANG, Hui BI, Yueni MEI, Shuo LIU, Yongji GONG, Wenlei LUO. Recent progress in high-energy and high-power lithium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(1): 54-76. |
| [6] | Xunchang JIANG, Kelin YU, Daxiang YANG, Minhui LIAO, Yang ZHOU. Preparation of PDOL-based solid electrolyte by in-situ polymerization and its application in lithium metal batteries [J]. Energy Storage Science and Technology, 2025, 14(1): 1-12. |
| [7] | Yijie YAO, Junwei ZHANG, Yanjun ZHAO, Hongcheng LIANG, Dongni ZHAO. Effect of interfacial dynamics on low temperature performance of sodium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(1): 30-41. |
| [8] | Junfeng HAO, Guanjun CEN, Ronghan QIAO, Jing ZHU, Qiangfu SUN, Xinxin ZHANG, Mengyu TIAN, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Hong ZHOU, Xuejie HUANG. Reviews of selected 100 recent papers for lithium batteries (Oct. 1, 2024 to Nov. 30, 2024) [J]. Energy Storage Science and Technology, 2025, 14(1): 388-405. |
| [9] | Ye TIAN, Shanshan WANG, Xu YAO, Jiaxin LIU, Xiaodong HAN. The significance and development of the collaborative application of distribution network communication and distributed energy storage technology [J]. Energy Storage Science and Technology, 2025, 14(1): 190-192. |
| [10] | Na WEN, Chengwei LIU, Xiaoyang ZHANG, Jian GAO, Liming MA. Research on the synergistic application of automation control and energy storage technology in smart grids [J]. Energy Storage Science and Technology, 2025, 14(1): 219-221. |
| [11] | Yuhang YUAN, Yuchen GAO, Jundong ZHANG, Yanbin GAO, Chaolong WANG, Xiang CHEN, Qiang ZHANG. The application of large language models in energy storage research [J]. Energy Storage Science and Technology, 2024, 13(9): 2907-2919. |
| [12] | Guobing ZHOU, Shenzhen XU. Progress of theoretical studies on the formation and growth mechanisms of solid electrolyte interphase at lithium metal anodes [J]. Energy Storage Science and Technology, 2024, 13(9): 3150-3160. |
| [13] | Xinxin ZHANG, Guanjun CEN, Ronghan QIAO, Jing ZHU, Junfeng HAO, Qiangfu SUN, Mengyu TIAN, Zhou JIN, Yuanjie ZHAN, Yong YAN, Liubin BEN, Hailong YU, Yanyan LIU, Hong ZHOU, Xueji HUANG. In-depth review of 100 pioneering studies on lithium batteries: Key innovations from June 1, 2024 to July 31, 2024 [J]. Energy Storage Science and Technology, 2024, 13(9): 3226-3244. |
| [14] | Jieyu ZHANG, Shun ZHANG, Ning LI, Fanglei ZENG, Jianning DING. Preparation and performance of a flame-retardant gel polymer electrolyte [J]. Energy Storage Science and Technology, 2024, 13(8): 2529-2540. |
| [15] | Chaofeng XU, Xiaolei HAN, Jinzhi WANG, Xiaojun WANG, Zhiming LIU, Jingwen ZHAO. Crystalline zinc-ion solid-state electrolytes based on weak coordination environments [J]. Energy Storage Science and Technology, 2024, 13(8): 2519-2528. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
