Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (8): 3122-3137.doi: 10.19799/j.cnki.2095-4239.2025.0289
• Energy Storage Materials and Devices • Previous Articles
Jingjing LI1( ), Danfeng JIANG2, Jiaxin LI2, Jie YAN2, Changjie SHEN3
), Danfeng JIANG2, Jiaxin LI2, Jie YAN2, Changjie SHEN3
Received:2025-03-27
Revised:2025-04-18
Online:2025-08-28
Published:2025-08-18
Contact:
Jingjing LI
E-mail:jjli@ipezz.ac.cn
CLC Number:
Jingjing LI, Danfeng JIANG, Jiaxin LI, Jie YAN, Changjie SHEN. Research progress on high specific-capacity lithium-rich single crystal materials[J]. Energy Storage Science and Technology, 2025, 14(8): 3122-3137.
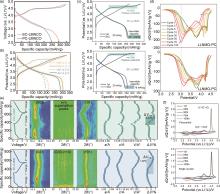
Fig. 2
Comparison of properties of PC and SC-LLNMO (a)[20], (b)[7], (c)[21]the charge and discharge curve, (d) 100 cycles dQ/dV[23], (e) in situ XRD (a and c are lattice constants and V represents the lattice volume,ΔVmaxrepresents the maximum volume change of the lattice during Li+ insertion process)[21], (f) dQ/dV[22]"
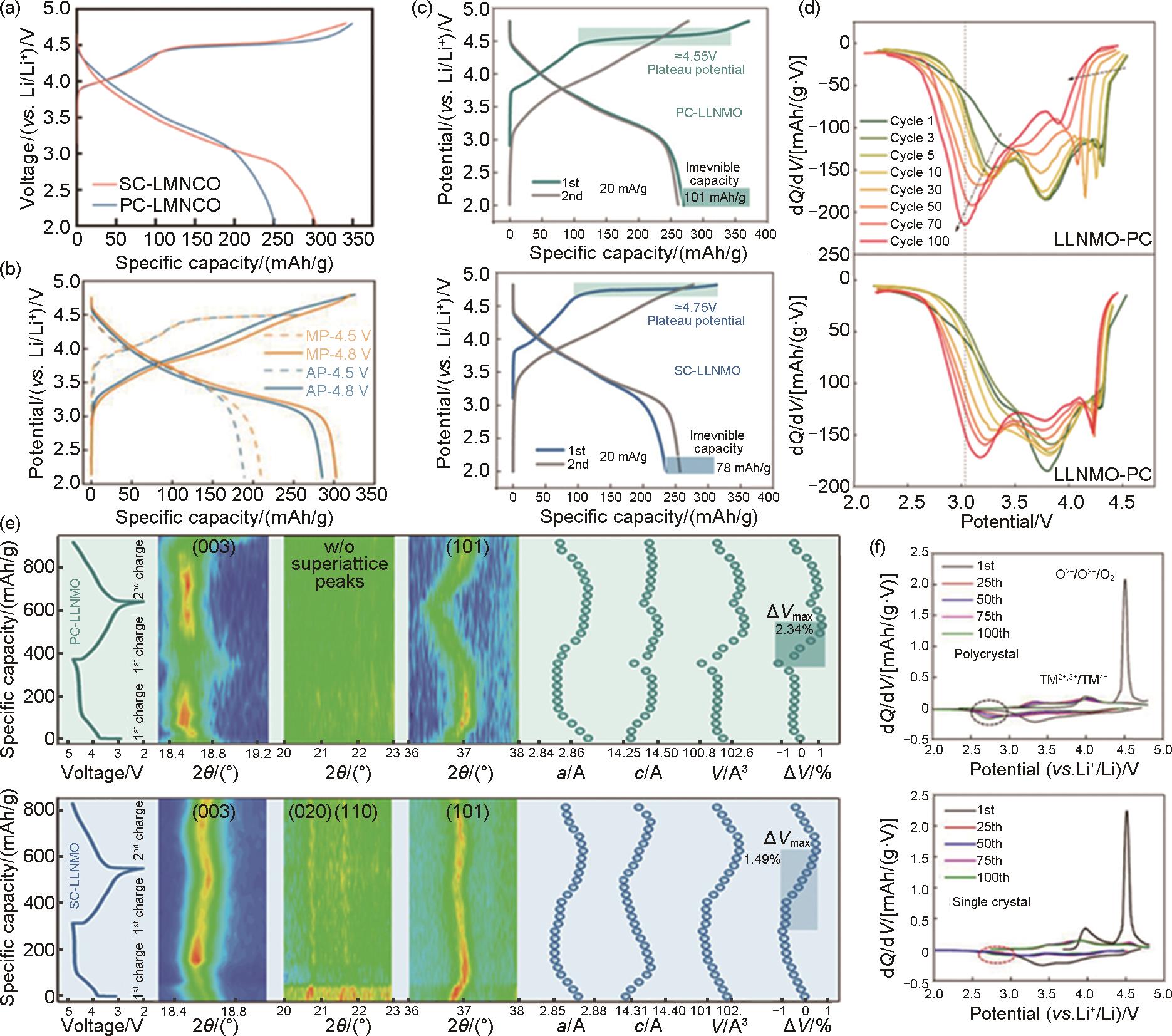
Table 1
Single crystal Li- rich samples prepared by different synthesis methods and electrochemical properties"
| 材料组成 | 合成方法 | 煅烧工艺 | 形貌 | 电压范围/V | 容量/(mAh/g) | 循环保持率/% | 文献 |
|---|---|---|---|---|---|---|---|
| Li1.2Mn0.54Ni0.13Co0.13O2 | 高温固相法 | 800*12 | 片状 | 2.0~4.8 | 254.5 | 71.9(1000) | [ |
| Li1.2Mn0.54Ni0.13Co0.13O2 | 高温固相法 | 450*6+900*10 | 不规则 | 2.0~4.8 | 291.4 | 89.8(100) | [ |
| Li1.1Na0.1Ni0.13Co0.13Mn0.54O2 | 高温固相法 | 450*5+850*12 | 不规则 | 2.0~4.8 | 278.5 | 87(100) | [ |
| Li1.2Mn0.54Ni0.13Co0.13O2 | 高温固相法 | 500*2+800*12 | 不规则 | 2.0~4.8 | 270 | 89(400) | [ |
| Li1.2Mn0.54Ni0.13Co0.13O2 | 高温固相法 | 450*5+900*12 | 不规则 | 2.0~4.8 | 290.4 | 100(100) | [ |
| Li1.2Ni0.13Co0.13Mn0.54O2 | 高温固相法 | 450*5+925*14 | 不规则 | 2.0~4.8 | 290.3 | 81.9(100) | [ |
| Li1.2Mn0.56Ni0.12Co0.12O2 | 溶剂热法 | 450*6+900*12 | 片状 | 2.0~4.8 | 306.9 | — | [ |
| 0.5Li2MnO3·0.5LiMn0.4Ni0.3Co0.3O2 | 溶剂热法 | 450*6+900*12 | 片状 | 2.0~4.8 | 300.1 | 93.5(50) | [ |
| Li1.2Mn0.56Co0.12Ni0.12O2 | 溶剂热法 | 900*12 | 纳米棒 | 2.0~4.8 | 264.6 | 91(100) | [ |
| Li1.2Ni0.13Co0.13Mn0.54O2 | 高温固相法 | 550*5+900*10+500*5 | 不规则 | 2.0~4.8 | 286.3 | 89(100) | [ |
| Li1.2Ni0.13Co0.13Mn0.54O2 | 高温固相法 | 500*5+850*15 | 片状 | 2.0~4.8 | 296 | 83.2(160) | [ |
| Li1.2Mn0.48Ni0.16Co0.16O2 | 高温固相法 | 940*2+760*10 | 不规则 | 2.0~4.8 | 259 | 84.9(100) | [ |
| Li1.2Mn0.533Ni0.267O2 | 熔融盐辅助法 | 500*5+900*20 | 细长状 | 2.0~4.8 | 240 | 84.06(200) | [ |
| Li1.2Mn0.56Ni0.16Co0.08O2 | 熔融盐辅助法 | 900*15 | 多边形 | 2.0~4.7 | 263.1 | 82.7(200) | [ |
| Li1.2Ni0.2Mn0.6O2 | 熔融盐辅助法 | 900*10 | 不规则 | 2.0~4.8 | 258 | 97.3(250) | [ |
| 0.5Li2MnO3· 0.5LiMn1/3Ni1/3Co1/3O2 | 熔融盐辅助法 | 900*12 | 不规则 | 2.0~4.8 | 268 | 82(100) | [ |
| Li1.2Mn0.533Ni0.267O2 | 熔融盐辅助法 | 500*5+900*20 | 不规则 | 2.0~4.8 | 210.8 | 84.06(200) | [ |
| Li1.2Ni0.13Mn0.54Co0.13O2 | 熔融盐辅助法 | 850*8 | 不规则 | 2.5~4.6 | 277 | — | [ |
| Li[Li0.2Ni0.2Mn0.6]O2 | 高温固相法 | 900*12 | 片状 | 2.0~4.8 | 253 | 85(200) | [ |
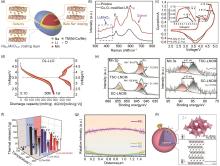
Fig. 7
(a) Structure diagram of sodium doped [28], (b) Raman spectrum of Co3O4 coated LR sample[34], (c) voltammetry diagram of LMN-P and LMN-C in the first week[63], (d) discharge curve DL-LLO cycle 30 times and corresponding dQ/dV[45], (e) XPS spectra of SC-LNCM and TSC-LNCM[64], (f) Lithium rich oxide particles with spherical secondary agglomerates (LLOs-SSA), LLOs-MCG and LLOs-MCG/400 at 4.4 V, 4.6 V and 4.8 V [50], (g) TM layer spacing, EDS, element curve and cyclic process dQ/dV[35], (h) LiErO2 coated crystal structure diagram[65]"
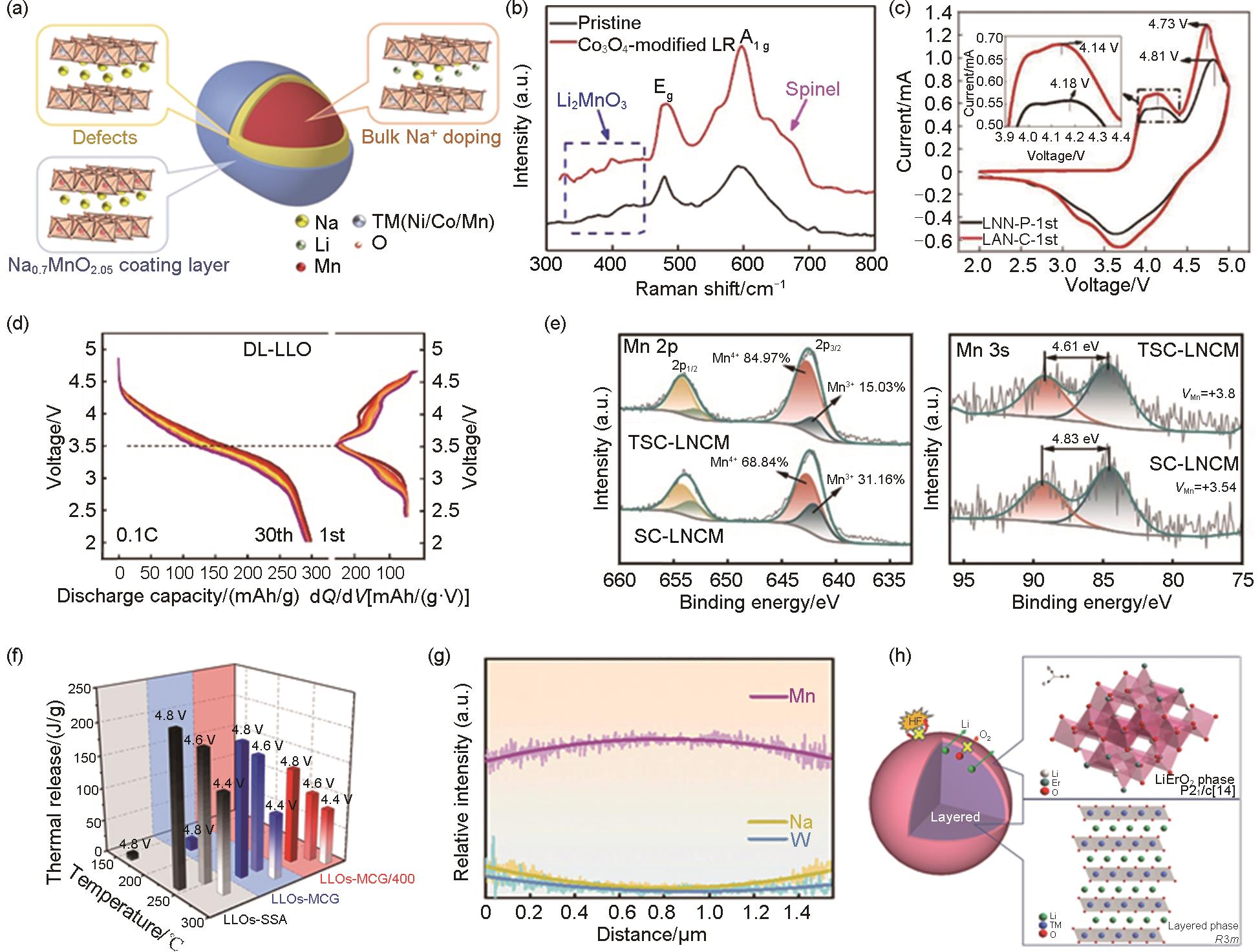

Fig. 8
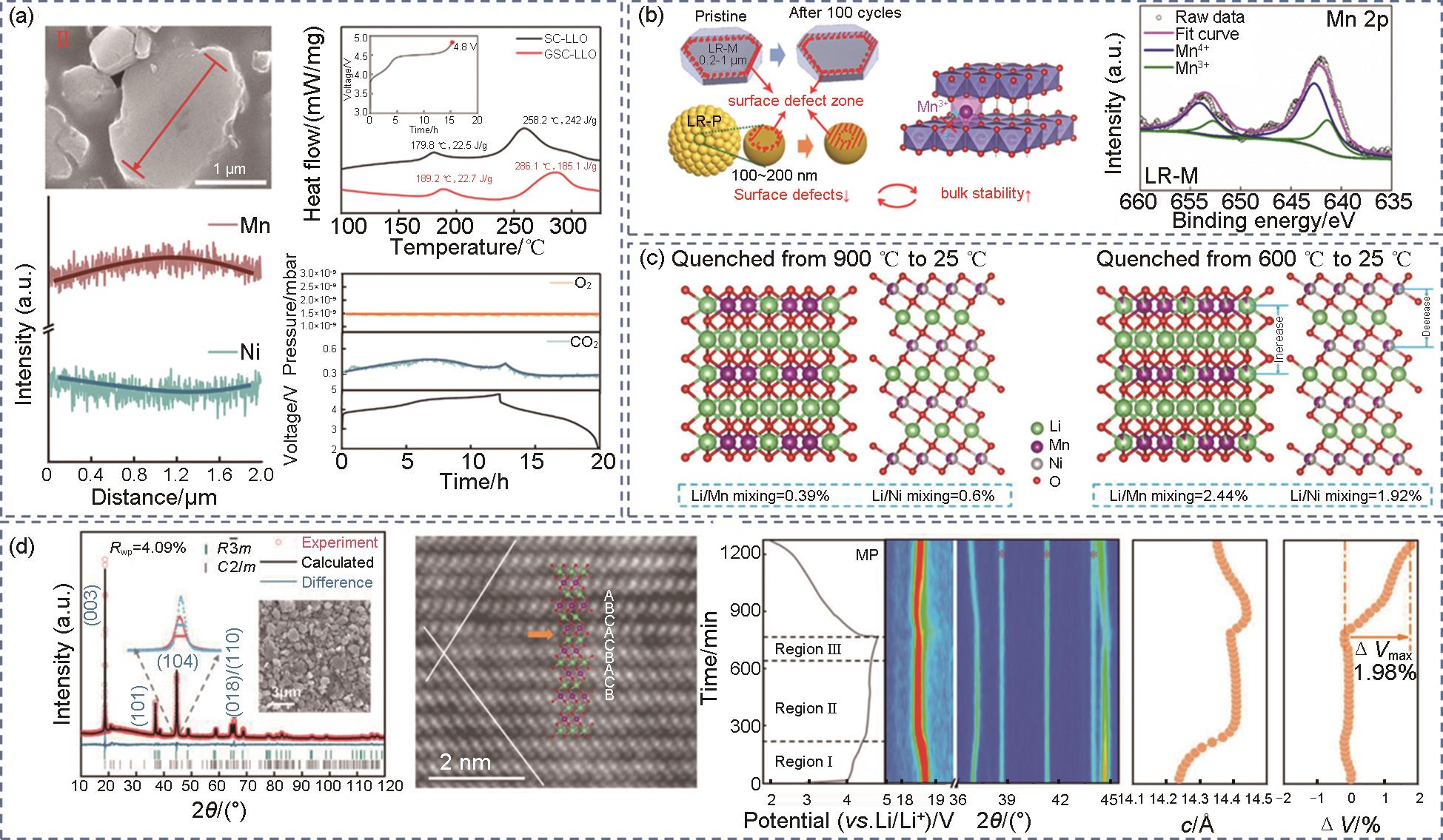
(a) SEM, Mn/Ni, DSC and in-situ DEMS of graded single crystal materials [48], (b) cyclic stability mechanism diagram and XPS after 100 cycles[25], (c) schematic diagram of Li2MnO3 and LiNi0.5Mn0.5O2[67], (d) XRD refinement pattern, where Rwp is the fitted R factor for the XRD refinement, iDPC-STEM, in-situ XRD[7]"
| [1] | CUI S L, GAO M Y, LI G R, et al. Insights into Li-rich Mn-based cathode materials with high capacity: From dimension to lattice to atom[J]. Advanced Energy Materials, 2022, 12(4): 2003885. DOI: 10.1002/aenm.202003885. |
| [2] | SHI J L, XIAO D D, GE M Y, et al. High-capacity cathode material with high voltage for Li-ion batteries[J]. Advanced Materials, 2018, 30(9): 1705575. DOI: 10.1002/adma.201705575. |
| [3] | LEE Y, LEE J, LEE K Y, et al. Facile formation of a Li3PO4 coating layer during the synthesis of a lithium-rich layered oxide for high-capacity lithium-ion batteries[J]. Journal of Power Sources, 2016, 315: 284-293. DOI: 10.1016/j.jpowsour.2016.03.024. |
| [4] | SHI J L, ZHANG J N, HE M, et al. Mitigating voltage decay of Li-rich cathode material via increasing Ni content for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(31): 20138-20146. DOI: 10.1021/acsami.6b06733. |
| [5] | ZHANG X D, HAO J J, WU L C, et al. Enhanced electrochemical performance of perovskite LaNiO3 coating on Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials for Li-ion batteries[J]. Electrochimica Acta, 2018, 283: 1203-1212. DOI: 10.1016/j.electacta.2018. 07.057. |
| [6] | ZHANG L J, LI N, WU B R, et al. Sphere-shaped hierarchical cathode with enhanced growth of nanocrystal planes for high-rate and cycling-stable li-ion batteries[J]. Nano Letters, 2015, 15(1): 656-661. DOI: 10.1021/nl5041594. |
| [7] | YANG Y L, GAO C, LUO T, et al. Unlocking the potential of Li-rich Mn-based oxides for high-rate rechargeable lithium-ion batteries[J]. Advanced Materials, 2023, 35(52): 2307138. DOI: 10.1002/adma.202307138. |
| [8] | LIU K L, ZHANG Q F, LU Z, et al. Molten-salt-assisted strategy enables high-rate micron-sized single-crystal Li-rich, Mn-based layered oxide cathode materials[J]. ACS Applied Materials & Interfaces, 2024, 16(12): 14902-14911. DOI: 10.1021/acsami. 4c00291. |
| [9] | SU Y F, WANG M, ZHANG M X, et al. The positive role of the single crystal morphology in improving the electrochemical performance of Li-rich cathode materials[J]. Journal of Alloys and Compounds, 2022, 905: 164204. DOI: 10.1016/j.jallcom.2022. 164204. |
| [10] | FAN X M, HU G R, ZHANG B, et al. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries[J]. Nano Energy, 2020, 70: 104450×6522: 1313-1317. DOI: 10.1126/science.abc3167. |
| [12] | LIU Y L, HARLOW J, DAHN J. Microstructural observations of "single crystal" positive electrode materials before and after long term cycling by cross-section scanning electron microscopy[J]. Journal of the Electrochemical Society, 2020, 167(2): 020512. DOI: 10.1149/1945-7111/ab6288. |
| [13] | SUN J M, CAO X, YANG H J, et al. The origin of high-voltage stability in single-crystal layered Ni-rich cathode materials[J]. Angewandte Chemie International Edition, 2022, 61(40): e2022 07225. DOI: 10.1002/anie.202207225. |
| [14] | LENG J, WANG J P, PENG W J, et al. Highly-dispersed submicrometer single-crystal nickel-rich layered cathode: Spray synthesis and accelerated lithium-ion transport[J]. Small, 2021, 17(14): 2006869. DOI: 10.1002/smll.202006869. |
| [15] | HU J T, LI L Z, BI Y J, et al. Locking oxygen in lattice: A quantifiable comparison of gas generation in polycrystalline and single crystal Ni-rich cathodes[J]. Energy Storage Materials, 2022, 47: 195-202. DOI: 10.1016/j.ensm.2022.02.025. |
| [16] | LOGAN E R, HEBECKER H, MA X W, et al. A comparison of the performance of different morphologies of LiNi0.8Mn0.1Co0.1O2 using isothermal microcalorimetry, ultra-high precision coulometry, and long-term cycling[J]. Journal of the Electrochemical Society, 2020, 167(6): 060530. DOI: 10.1149/1945-7111/ab8620. |
| [17] | HU J T, WANG H B, XIAO B W, et al. Challenges and approaches of single-crystal Ni-rich layered cathodes in lithium batteries[J]. National Science Review, 2023, 10(12): nwad252. DOI: 10.1093/nsr/nwad252. |
| [18] | CHA H, KIM J, LEE H, et al. Boosting reaction homogeneity in high-energy lithium-ion battery cathode materials[J]. Advanced Materials, 2020, 32(39): 2003040. DOI: 10.1002/adma.202003040. |
| [19] | ZHAO X W, CAO X, SHENG C C, et al. Perspective on high-stability single-crystal Li-rich cathode materials for Li-ion batteries[J]. ACS Applied Materials & Interfaces, 2024, 16(19): 24147-24161. DOI: 10.1021/acsami.4c05206. |
| [20] | LI L T, CHEN Y F, LIU Y C, et al. Synthesis of high-performance single-crystal Li-rich cathode by self-combustion method[J]. Rare Metals, 2023, 42(3): 830-837. DOI: 10.1007/s12598-022-02158-z. |
| [21] | SUN J M, SHENG C C, CAO X, et al. Restraining oxygen release and suppressing structure distortion in single-crystal Li-rich layered cathode materials[J]. Advanced Functional Materials, 2022, 32(10): 2110295. DOI: 10.1002/adfm.202110295. |
| [22] | HAO Z K, GOU X X, MA H Y, et al. Boosting the cycle and rate performance of Li1.2Mn0.54Ni0.13Co0.13O2 via single-crystal structure design[J]. Science China Materials, 2023, 66(9): 3424-3432. DOI: 10.1007/s40843-023-2494-1. |
| [23] | YANG X X, WANG S N, HAN D Z, et al. Structural origin of suppressed voltage decay in single-crystalline Li-rich layered Li [Li0.2Ni0.2Mn0.6]O2 cathodes[J]. Small, 2022, 18(25): 2201522. DOI: 10.1002/smll.202201522. |
| [24] | YOON M S, DONG Y H, HUANG Y M, et al. Eutectic salt-assisted planetary centrifugal deagglomeration for single-crystalline cathode synthesis[J]. Nature Energy, 2023: 482-491. |
| [25] | XIE Y, MENG S, CHEN X, et al. Synergetic effect of high Ni ratio and low oxygen defect interface zone of single crystals on the capacity retention of lithium rich layered oxides[J]. Journal of Colloid and Interface Science, 2021, 594: 485-492. DOI: 10.1016/j.jcis.2021.03.038. |
| [26] | HE W X, LIU J G, SUN W, et al. Coprecipitation-gel synthesis and degradation mechanism of octahedral Li1.2Mn0.54Ni0.13Co0.13O2 as high-performance cathode materials for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(27): 23018-23028. DOI: 10.1021/acsami.8b04023. |
| [27] | XU C Y, LI J L, SUN J, et al. Li-rich layered oxide single crystal with Na doping as a high-performance cathode for Li ion batteries[J]. Journal of Alloys and Compounds, 2022, 895: 162613. DOI: 10.1016/j.jallcom.2021.162613. |
| [28] | LIU Q, XIE T, XIE Q S, et al. Multiscale deficiency integration by Na-rich engineering for high-stability Li-rich layered oxide cathodes[J]. ACS Applied Materials & Interfaces, 2021, 13(7): 8239-8248. DOI: 10.1021/acsami.0c19040. |
| [29] | LIN S Z, WANG Z, LU T X, et al. One-step preparation of homogeneous single crystal Li-rich cathode materials with encouraging electrochemical performance[J]. Journal of Alloys and Compounds, 2020, 822: 153638. DOI: 10.1016/j.jallcom. 2020.153638. |
| [30] | 缪胤宝, 张文华, 刘伟昊, 等. 富锂正极材料Li1.2Ni0.13Co0.13Mn0.54O2的制备及性能[J]. 储能科学与技术, 2024, 13(5): 1427-1434. DOI: 10.19799/j.cnki.2095-4239.2023.0850. |
| [31] | FU F, HUANG Y Y, WU P, et al. Controlled synthesis of lithium-rich layered Li1.2Mn0.56Ni0.12Co0.12O2 oxide with tunable morphology and structure as cathode material for lithium-ion batteries by solvo/hydrothermal methods[J]. Journal of Alloys and Compounds, 2015, 618: 673-678. DOI: 10.1016/j.jallcom.2014.08.191 |
| [32] | FU F, DENG Y P, SHEN C H, et al. A hierarchical micro/nanostructured 0.5Li2MnO3·0.5LiMn0.4Ni0.3Co0.3O2 material synthesized by solvothermal route as high rate cathode of lithium ion battery[J]. Electrochemistry Communications, 2014, 44: 54-58. DOI: 10.1016/j.elecom.2014.04.013. |
| [33] | FU F, YAO Y Z, WANG H Y, et al. Structure dependent electrochemical performance of Li-rich layered oxides in lithium-ion batteries[J]. Nano Energy, 2017, 35: 370-378. DOI: 10.1016/j.nanoen.2017.04.005. |
| [34] | XIN Y, LAN X W, CHANG P, et al. Conformal spinel/layered heterostructures of Co3O4 shells grown on single-crystal Li-rich nanoplates for high-performance lithium-ion batteries[J]. Applied Surface Science, 2018, 447: 829-836. DOI: 10.1016/j.apsusc. 2018.04.070. |
| [35] | DUAN J D, WANG F Q, HUANG M J, et al. High-performance single-crystal lithium-rich layered oxides cathode materials via Na2WO4-assisted sintering[J]. Small, 2024, 20(15): 2307998. DOI: 10.1002/smll.202307998. |
| [36] | SUN J M, CAO X, YANG W H, et al. Impact of particle size on the kinetics and structure stability of single-crystal Li-rich cathode materials[J]. Journal of Materials Chemistry A, 2023, 11(26): 13956-13964. DOI: 10.1039/D3TA01624B. |
| [37] | ZHONG X X, CHEN M, ZHU Y M, et al. Layered lithium-rich oxide nanoparticles: Low-temperature synthesis in mixed molten salt and excellent performance as cathode of lithium-ion battery[J]. Ionics, 2017, 23(8): 1955-1966. DOI: 10.1007/s11581-017-2039-4. |
| [38] | KUPPAN S, SHUKLA A K, MEMBRENO D, et al. Revealing anisotropic spinel formation on pristine Li- and Mn-rich layered oxide surface and its impact on cathode performance[J]. Advanced Energy Materials, 2017, 7(11): 1602010. DOI: 10.1002/aenm.201602010. |
| [39] | JIAO C M, WANG M, HUANG B, et al. Surface modification single crystal Li-rich Li1.2Mn0.54Ni0.13Co0.13O2 as high performance cathode materials for Li-ion batteries[J]. Journal of Alloys and Compounds, 2023, 937: 168389. DOI: 10.1016/j.jallcom.2022. 168389. |
| [40] | LI J, LI H Y, STONE W, et al. Synthesis of single crystal LiNi0.5Mn0.3Co0.2O2 for lithium ion batteries[J]. Journal of the Electrochemical Society, 2017, 164(14): A3529-A3537. DOI: 10. 1149/2.0401714jes. |
| [41] | ZHANG B D, ZHANG Y M, WU H C, et al. Does single-crystallization a feasible direction for designing Li-rich layered cathodes [J]. Energy Storage Materials, 2023, 62: 102926. DOI: 10.1016/j.ensm.2023.102926. |
| [42] | ZHAO X W, SHENG C C, CHANG Z, et al. Solid-state exfoliation growth mechanism of single-crystal Li-rich layered cathode materials[J]. Energy Storage Materials, 2025, 75: 104093. DOI: 10.1016/j.ensm.2025.104093. |
| [43] | YAN L Y, GAO Y, CHEN M M, et al. Preparation of single-crystal Li-rich Mn-based layered oxides with excellent electrochemical performance via simple stepwise high-temperature sintering[J]. ACS Applied Materials & Interfaces, 2024, 16(44): 60166-60179. DOI: 10.1021/acsami.4c11204. |
| [44] | ZHAO Z Y, HUANG B, WANG M, et al. Facile synthesis of fluorine doped single crystal Ni-rich cathode material for lithium-ion batteries[J]. Solid State Ionics, 2019, 342: 115065. DOI: 10. 1016/j.ssi.2019.115065. |
| [45] | ZENG W H, LIU F, YANG J L, et al. Single-crystal Li-rich layered cathodes with suppressed voltage decay by double-layer interface engineering[J]. Energy Storage Materials, 2023, 54: 651-660. DOI: 10.1016/j.ensm.2022.11.016. |
| [46] | LIU T C, LIU J J, LI L X, et al. Origin of structural degradation in Li-rich layered oxide cathode[J]. Nature, 2022, 606(7913): 305-312. DOI: 10.1038/s41586-022-04689-y. |
| [47] | HE H C, LI H, MOU L S, et al. Achieving long cycle life and single-crystal of Li-rich Mn-based cathodes by reheating with sintering additives[J]. Journal of Physics: Conference Series, 2023, 2539(1): 012040. DOI: 10.1088/1742-6596/2539/1/012040. |
| [48] | WU T H, ZHANG X, WANG Y Z, et al. Gradient "single-crystal" Li-rich cathode materials for high-stable lithium-ion batteries[J]. Advanced Functional Materials, 2023, 33(4): 2210154. DOI: 10. 1002/adfm.202210154. |
| [49] | WANG L, XU L, XUE W R, et al. Synergistic enhancement of Li-rich manganese-based cathode materials through single crystallization and in situ spinel coating[J]. Nano Energy, 2024, 121: 109241. DOI: 10.1016/j.nanoen.2023.109241. |
| [50] | WANG Y Z, WANG L, GUO X W, et al. Thermal stability enhancement through structure modification on the microsized crystalline grain surface of lithium-rich layered oxides[J]. ACS Applied Materials & Interfaces, 2020, 12(7): 8306-8315. DOI: 10. 1021/acsami.9b21303. |
| [51] | ZUO P, WANG F X, CHEN G Y, et al. Facet-dependent Ni segregation in a micron-sized single-crystal Li1.2Ni0.2Mn0.6O2 cathode[J]. ACS Applied Materials & Interfaces, 2024. DOI: 10. 1021/acsami.4c02885. |
| [52] | PENG H, ZHAO S X, HUANG C, et al. In situ construction of spinel coating on the surface of a lithium-rich manganese-based single crystal for inhibiting voltage fade[J]. ACS Applied Materials & Interfaces, 2020, 12(10): 11579-11588. DOI: 10.1021/acsami. 9b21271. |
| [53] | LI S F, QIAN G N, HE X M, et al. Thermal-healing of lattice defects for high-energy single-crystalline battery cathodes[J]. Nature Communications, 2022, 13: 704. DOI: 10.1038/s41467-022-28325-5. |
| [54] | LI X Q, ZHOU L M, WANG H, et al. Dopants modulate crystal growth in molten salts enabled by surface energy tuning[J]. Journal of Materials Chemistry A, 2021, 9(35): 19675-19680. DOI: 10.1039/D1TA02351A. |
| [55] | CHEN G Y, HAI B, SHUKLA A K, et al. Impact of initial Li content on kinetics and stabilities of layered Li1+ x(Ni0.33Mn0.33Co0.33)1+ xO2[J]. Journal of the Electrochemical Society, 2012, 159(9): A1543-A1550. DOI: 10.1149/2.038209jes. |
| [56] | MIAO X W, YAN Y, WANG C G, et al. Optimal microwave-assisted hydrothermal synthesis of nanosized xLi2MnO3·(1-x)LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium ion battery[J]. Journal of Power Sources, 2014, 247: 219-227. DOI: 10.1016/j.jpowsour.2013.08.097. |
| [57] | HU S J, LI Y, CHEN Y H, et al. Insight of a phase compatible surface coating for long-durable Li-rich layered oxide cathode[J]. Advanced Energy Materials, 2019, 9(34): 1901795. DOI: 10.1002/aenm.201901795. |
| [58] | WEI Y R, CHENG J, LI D P, et al. A structure self-healing Li-rich cathode achieved by lithium supplement of Li-rich LLZO coating[J]. Advanced Functional Materials, 2023, 33(22): 2214775. DOI: 10.1002/adfm.202214775. |
| [59] | SHAO Q N, GAO P Y, YAN C H, et al. A redox couple strategy enables long-cycling Li- and Mn-rich layered oxide cathodes by suppressing oxygen release[J]. Advanced Materials, 2022, 34(14): 2108543. DOI: 10.1002/adma.202108543. |
| [60] | YU R Z, WANG C H, DUAN H, et al. Manipulating charge-transfer kinetics of lithium-rich layered oxide cathodes in halide all-solid-state batteries[J]. Advanced Materials, 2023, 35(5): 2207234. DOI: 10.1002/adma.202207234. |
| [61] | LI J L, LIN H Y, TANG C J, et al. Na doping into Li-rich layered single crystal nanoparticles for high-performance lithium-ion batteries cathodes[J]. Nanotechnology, 2022, 33(6): 065705. DOI: 10.1088/1361-6528/ac353c. |
| [62] | YALÇıN A, DEMIR M, GÜLER M O, et al. Synthesis of Sn-doped Li-rich NMC as a cathode material for Li-ion batteries[J]. Electrochimica Acta, 2023, 440: 141743. DOI: 10.1016/j.electacta. 2022.141743. |
| [63] | LIU Y L, LI B, CHEN M, et al. Low-temperature-aged synthesis of CeO2-coated Li-rich oxide as cathode for low-cost high-energy density Li-ion batteries[J]. Batteries, 2023, 9(6): 330. DOI: 10. 3390/batteries9060330. |
| [64] | DUAN J D, WANG F Q, LI S M, et al. Stable surface lattice for prolonged cycle life of single-crystal lithium-rich layered oxide cathodes[J]. Chemical Engineering Journal, 2024, 489: 151257. DOI: 10.1016/j.cej.2024.151257. |
| [65] | WEI Z C, ZHANG D, ZHONG J J, et al. Enhanced cycling stability of lithium-rich cathode materials achieved by in situ formation of LiErO2 coating[J]. Batteries & Supercaps, 2023, 6(4): e202200568. DOI: 10.1002/batt.202200568. |
| [66] | ZHU Z, YU D W, YANG Y, et al. Gradient Li-rich oxide cathode particles immunized against oxygen release by a molten salt treatment[J]. Nature Energy, 2019, 4(12): 1049-1058. DOI: 10. 1038/s41560-019-0508-x. |
| [67] | CAI Z F, WANG S, ZHU H K, et al. Improvement of stability and capacity of co-free, Li-rich layered oxide Li1.2Ni0.2Mn0.6O2 cathode material through defect control[J]. Journal of Colloid and Interface Science, 2023, 630(Pt B): 281-289. DOI: 10.1016/j.jcis.2022. 10.105. |
| [68] | CELESTE A, TUCCILLO M, SANTONI A, et al. Exploring a co-free, Li-rich layered oxide with low content of nickel as a positive electrode for Li-ion battery[J]. ACS Applied Energy Materials, 2021, 4(10): 11290-11297. DOI: 10.1021/acsaem.1c02133. |
| [1] | Zhoulan ZENG, Lei SHANG, Zhijin HU, Zongfan WANG, Xiaochao XIN, Ying LIU. Li5FeO4@C high capacity prelithium cathode materials for lithium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(5): 1875-1883. |
| [2] | Shuaijing JI, Junwei WANG, Baoshuai DU, Li XU, Ping LOU, Minyuan GUAN, Shun TAN, Shijie CHENG, Yuancheng CAO. Improvement paths for the stability and safety of LiFe x Mn1–x PO4 (0 < x < 1) batteries: From failure mechanisms to comprehensive optimization strategies [J]. Energy Storage Science and Technology, 2025, 14(3): 965-983. |
| [3] | Xinyuan JIA, Xianfu ZHANG, Long ZHANG. Research progress on micromodification and macrodesign of Zn powder anodes in aqueous Zn metal batteries [J]. Energy Storage Science and Technology, 2025, 14(3): 913-929. |
| [4] | Deqing ZHOU, Yijia CAI, Ziqin ZHANG, Liping ZHOU, Sijiang HU, Youguo HUANG, Hongqiang WANG, Qingyu LI. Electrochemical properties of spinel MoS2 coated lithium-rich manganese-based cathode materials [J]. Energy Storage Science and Technology, 2025, 14(3): 1087-1096. |
| [5] | Zhiyong WANG, Junyao CAI, Yingqi SHE, Shulin ZHONG, Kanghua PAN. Surface-modification of graphite with N-heterocyclic conducting polymers as high performance anodes for Li-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(8): 2511-2518. |
| [6] | Dingbang HAO, Yongli LI. Na0.85Ni0.3Fe0.2Mn0.5O1.95F0.05@CuO cathode materials for high-rate and long cycling stability sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(8): 2489-2498. |
| [7] | Weiqi LIN, Qiaoyu LU, Yuhong CHEN, Linyuan QIU, Yurong JI, Lianyu GUAN, Xiang DING. Advances in cathode materials for low-temperature sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2348-2360. |
| [8] | Pengfei XIAO, Lin MEI, Libao CHEN. Multicomponent-coated graphite composite anodes for low-temperature electrochemical energy storage [J]. Energy Storage Science and Technology, 2024, 13(7): 2116-2123. |
| [9] | Boyu LIU, Qing PANG, Tengfei WANG, Hongyu WANG. Advancements in the modification of high-voltage Ni-rich ternary cathode material LiNi0.8Co0.1Mn0.1O2 for lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(11): 3784-3795. |
| [10] | Zhen CHEN, Xian'ao LI, Yiwei XU, Xin LIU, Zexiang SHEN, Minghua CHEN. Current research status and future prospects of the synthesis and modification routes for LATP and LAGP solid electrolytes [J]. Energy Storage Science and Technology, 2024, 13(11): 3826-3855. |
| [11] | Zeping FANG, Bao QIU, Zhaoping LIU. Progress of "reversible high-oxygen activity" of lithium-rich layered oxide anode materials [J]. Energy Storage Science and Technology, 2024, 13(1): 240-251. |
| [12] | Yayun LIAO, Feng ZHOU, Yingxi ZHANG, Tu'an LV, Yang HE, Xiaoyan CHEN, Kaifu HUO. Research progress on fast-charging graphite anode materials for lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(1): 130-142. |
| [13] | Jilu ZHANG, Yuchen DONG, Qiang SONG, Siming YUAN, Xiaodong GUO. Controllable synthesis and electrochemical mechanism related to polycrystalline and single-crystalline Ni-rich layered LiNi0.9Co0.05Mn0.05O2 cathode materials [J]. Energy Storage Science and Technology, 2023, 12(8): 2382-2389. |
| [14] | Zhengguang ZHAO, Zhenying CHEN, Guangqun ZHAI, Xi ZHANG, Xiaodong ZHUANG. Preparation of Sc/O-doped sulfide electrolyte for all-solid-state batteries [J]. Energy Storage Science and Technology, 2023, 12(8): 2412-2423. |
| [15] | Kangkang QU, Yahua LIU, Die HONG, Zhaoxi SHEN, Xiaozhao HAN, Xu ZHANG. Research progress on positive electrolytes for neutral aqueous organic redox flow battery [J]. Energy Storage Science and Technology, 2023, 12(5): 1570-1588. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
