Energy Storage Science and Technology ›› 2025, Vol. 14 ›› Issue (2): 525-543.doi: 10.19799/j.cnki.2095-4239.2024.0663
• Energy Storage Materials and Devices • Previous Articles Next Articles
Lishuai ZHANG( ), Yifei ZHANG, Yiyang MA, Sibo ZHAO, Hongquan LIU, Shengting SHI, Yanjun ZHONG(
), Yifei ZHANG, Yiyang MA, Sibo ZHAO, Hongquan LIU, Shengting SHI, Yanjun ZHONG( )
)
Received:2024-07-17
Revised:2024-08-03
Online:2025-02-28
Published:2025-03-18
Contact:
Yanjun ZHONG
E-mail:3185414642@qq.com;yjzhong@scu.edu.cn
CLC Number:
Lishuai ZHANG, Yifei ZHANG, Yiyang MA, Sibo ZHAO, Hongquan LIU, Shengting SHI, Yanjun ZHONG. Research progress on sodium-ion battery cathode materials based on iron-based prussian blue analogues[J]. Energy Storage Science and Technology, 2025, 14(2): 525-543.
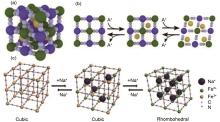
Fig.1
Prussian Blue Analogs (PBAs) face-centered cubic geometry and open framework structure (a)[31],when the oxidation state of transition metal ions changes, the insertion of alkali metal ions into the sub-cuboids of the lattice causes structural changes[31], redox mechanism and phase transition schematic of Fe-PBAs (c)[32]"
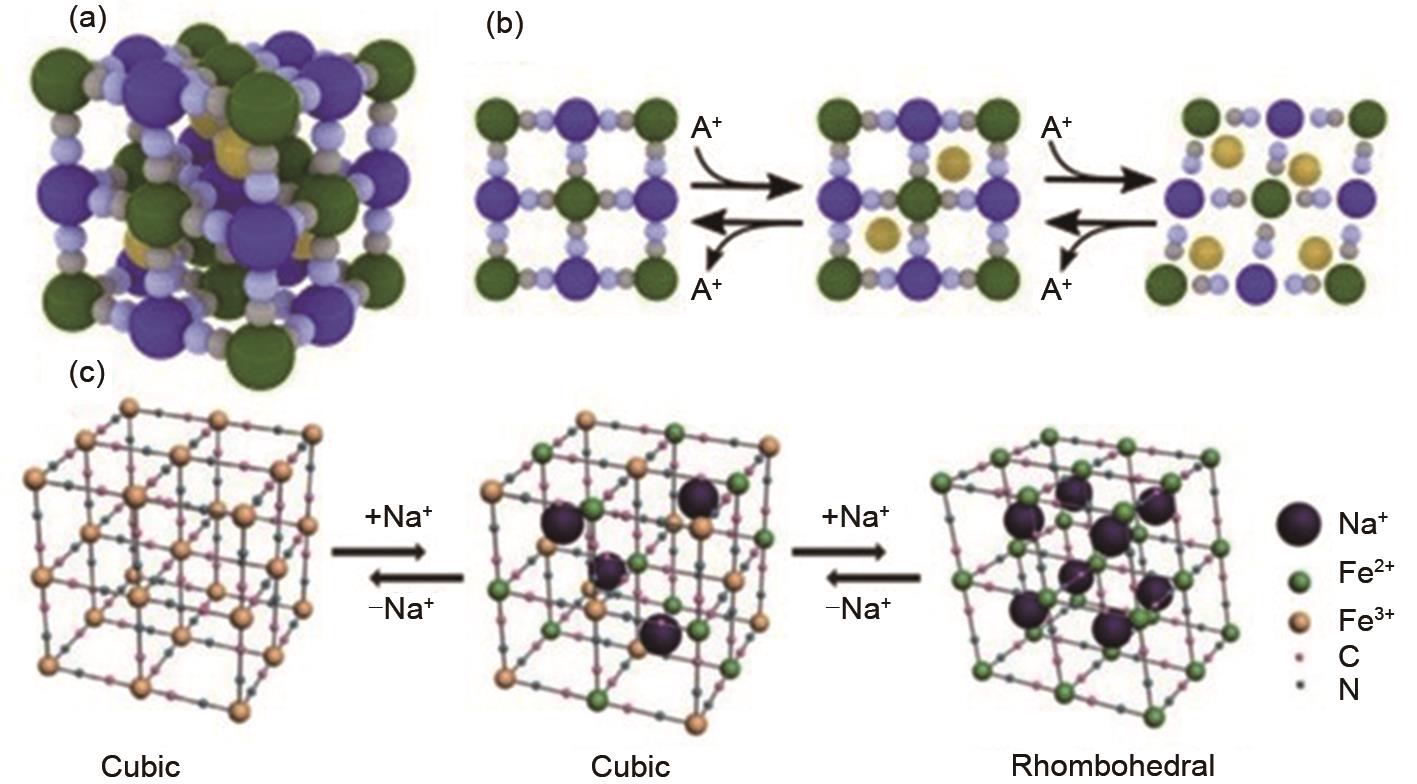
Figure 5
(a) Rietveld refinement analysis of PBA(Cu)□Fe, (b) SEM image of PBA(Cu)□Feg), (c) rate performance, and (d) cycling performance comparison between PBA, PBA□Fe, and PBA(Cu)□Fe at 500 mA/g[107], (e) In situ XRD result of ZnFeHCF-2. Electrochemical performance of ZnFeHCF-2/hard carbon full cell (f) cycling performance, (g) Rate performance[108]"
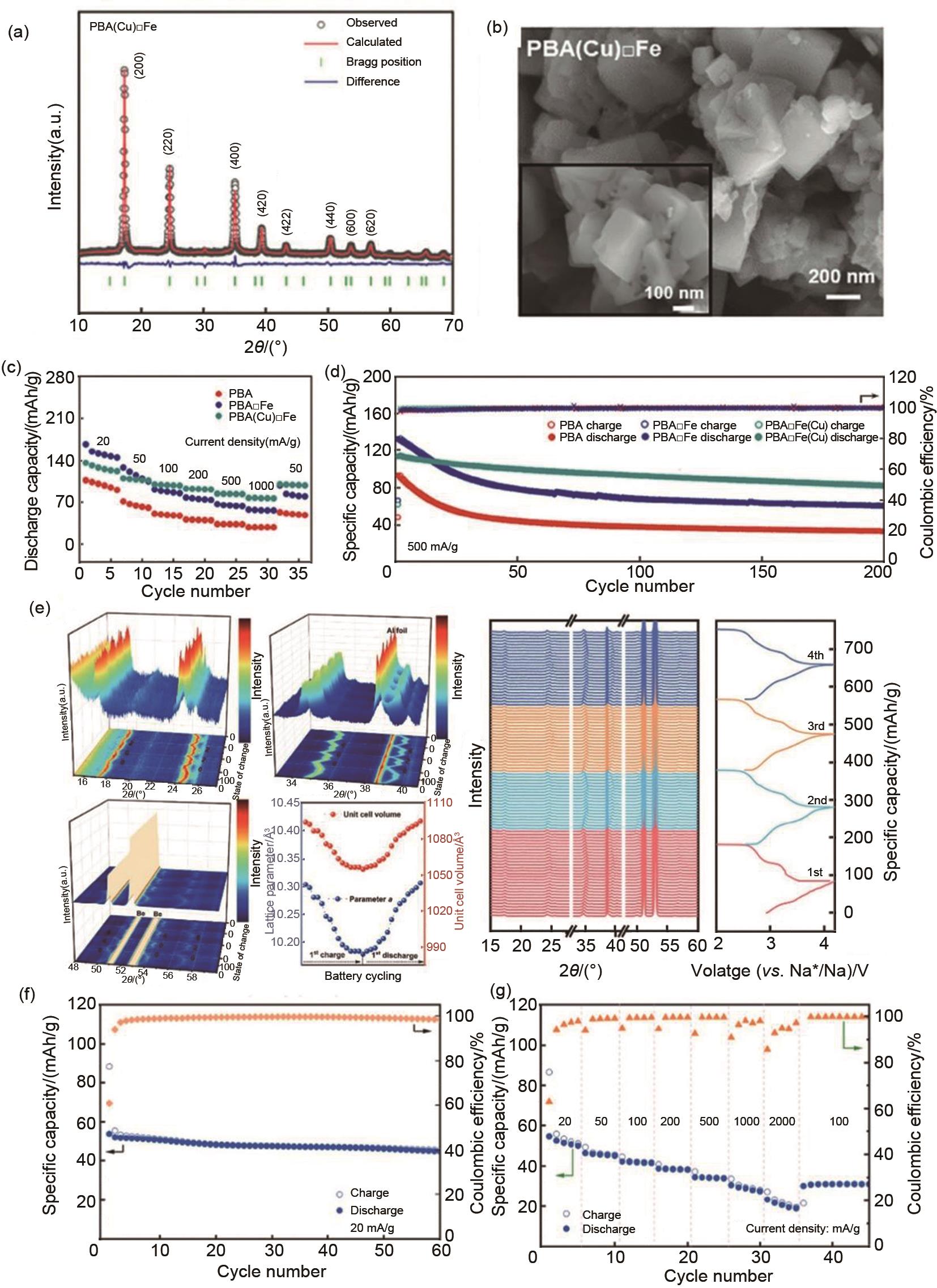

Fig.8
Schematic illustration of the synthetic process for PB@ZnO (a); SEM image (b) and TEM image (c) of PB@ZnO; charge and discharge curves for PB and PB@ZnO at 0.2 A/g in the first cycle (d); (e) rate performance of PB@ZnO and PB at different current densities; cycling performance of PB@ZnO and PB at 0.2 A/g(f) [40]"
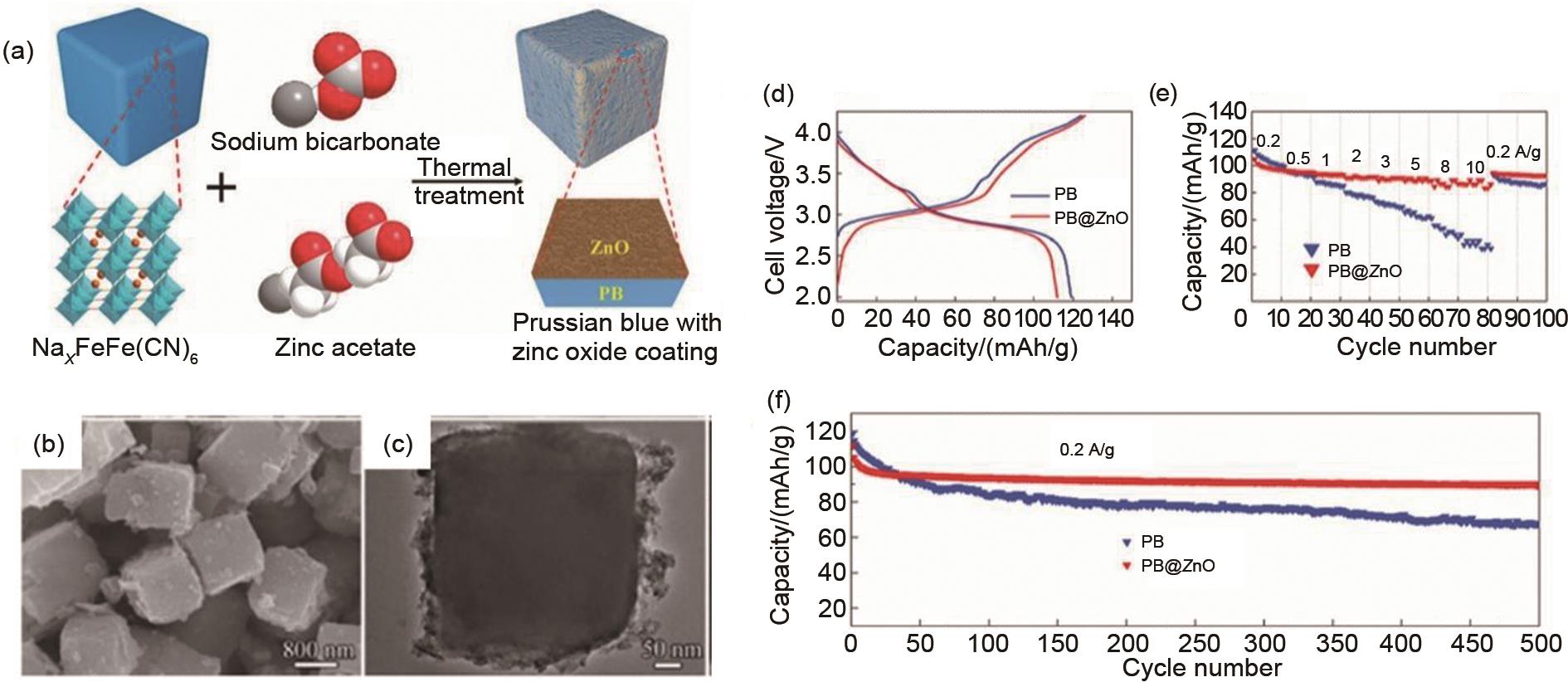
Table 1
Comparison of sodium storage performance of Fe-PBAs in electrolytes with different compositions"
| 电解液组成 | 电压范围/V | 比容量/(mAh/g) | 电流密度/(mA/g) | 循环性能 | 文献 |
|---|---|---|---|---|---|
| 1.0 mol/L NaPF6 in EC/PC (1∶1, 体积比) | 2.0~4.2 | 131, 99.8 | 17 340 | 200次,82.9% (170 mA/g) | [ |
| 1.0 mol/L NaPF6 in PC/EMC/FEC/亚硫酸丙烯酯(PST)/亚硫酸乙烯酯(DTD) (40∶58∶2∶1∶1, 体积比) | 2.0~4.0 | 106.5 | 170 | 500次,96.7% (170 mA/g) | [ |
| 1.0 mol/L NaClO4 in EC/PC (1∶1, 体积比) | 2.0~3.9 | 58.3 | 1000 | 1000次,59.7% (1000 mA/g) | [ |
| 1 mol/L NaClO4 in EC/DMC = (1∶1) with 1% (体积分数) FEC | 2.0~4.0 | 105 | 200 | 1000次,69.1% (200 mA/g) | [ |
| 1.0 mol/L NaClO4 in DEC/EC/FEC (1∶1∶0.05, 体积比) | 2.0~4.0 | 145.3 | 34 | 1000次,54.5% (34 mA/g) | [ |
| 1 mol/L NaPF6 in PC with 10% (体积分数) FEC | 2.0~4.2 | 96.8 | 9000 | 500次,61.6% (500 mA/g) | [ |
| 1 mol/L NaPF6 in EC/DEC (1∶1, 体积比) | 2.0~4.2 | 115 | 50 | 150次,96% (50 mA/g) | [ |
| 1 mol/L NaClO4 in EC/DMC/EMC (1∶1∶1, 体积比) with 5% (体积分数) FEC | 2.0~4.2 | 113 | 1600 | 400次,80% (800 mA/g) | [ |
| 1 | DETKA K, GÓRECKI K. Selected technologies of electrochemical energy storage—a review[J]. Energies, 2023, 16(13): 5034. DOI: 10.3390/en16135034. |
| 2 | KIM T, SONG W T, SON D Y, et al. Lithium-ion batteries: Outlook on present, future, and hybridized technologies[J]. Journal of Materials Chemistry A, 2019, 7(7): 2942-2964. DOI: 10.1039/C8TA10513H. |
| 3 | FANG C, HUANG Y H, ZHANG W X, et al. Routes to high energy cathodes of sodium-ion batteries[J]. Advanced Energy Materials, 2016, 6(5): 1501727. DOI: 10.1002/aenm.201501727. |
| 4 | HAN Q G, LI X, WANG F X, et al. Carbon fiber@ pore-ZnO composite as anode materials for structural lithium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2019, 833: 39-46. DOI: 10.1016/j.jelechem.2018.11.014. |
| 5 | LI M, LU J, CHEN Z W, et al. 30 years of lithium-ion batteries[J]. Advanced Materials, 2018: e1800561. DOI: 10.1002/adma. 201800561. |
| 6 | ABRAHAM K M. How comparable are sodium-ion batteries to lithium-ion counterparts?[J]. ACS Energy Letters, 2020, 5(11): 3544-3547. DOI: 10.1021/acsenergylett.0c02181. |
| 7 | LIU T F, ZHANG Y P, JIANG Z G, et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage[J]. Energy & Environmental Science, 2019, 12(5): 1512-1533. DOI: 10.1039/C8EE03727B. |
| 8 | USISKIN R, LU Y X, POPOVIC J, et al. Fundamentals, status and promise of sodium-based batteries[J]. Nature Reviews Materials, 2021, 6: 1020-1035. DOI: 10.1038/s41578-021-00324-w. |
| 9 | CHA S, KIM C, KIM H, et al. Electrochemical properties of micro-sized bismuth anode for sodium ion batteries[J]. Science of Advanced Materials, 2020, 12(9): 1429-1432. DOI: 10.1166/sam.2020.3801. |
| 10 | LI Y, WU F, LI Y, et al. Ether-based electrolytes for sodium ion batteries[J]. Chemical Society Reviews, 2022, 51(11): 4484-4536. DOI: 10.1039/D1CS00948F. |
| 11 | NAYAK P K, YANG L T, BREHM W, et al. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises[J]. Angewandte Chemie (International Ed), 2018, 57(1): 102-120. DOI: 10.1002/anie.201703772. |
| 12 | HASA I, MARIYAPPAN S, SAUREL D, et al. Challenges of today for Na-based batteries of the future: From materials to cell metrics[J]. Journal of Power Sources, 2021, 482: 228872. DOI: 10.1016/j.jpowsour.2020.228872. |
| 13 | 张洪霞, 李少芳, 赵博, 等. 钠离子电池用铁基正极材料的研究进展[J]. 无机化学学报, 2020, 36(7): 1205-1222. DOI: 10.11862/CJIC.2020.136. |
| ZHANG H X, LI S F, ZHAO B, et al. Research progresses on iron-based cathode materials for sodium-ion batteries[J]. Chinese Journal of Inorganic Chemistry, 2020, 36(7): 1205-1222. DOI: 10.11862/CJIC.2020.136. | |
| 14 | CHEN X, FENG X, REN B, et al. High rate and long lifespan sodium-organic batteries using pseudocapacitive porphyrin complexes-based cathode[J]. Nano-Micro Letters, 2021, 13(1): 71. DOI: 10.1007/s40820-021-00593-8. |
| 15 | ZHANG H, GAO Y, LIU X H, et al. Long-cycle-life cathode materials for sodium-ion batteries toward large-scale energy storage systems[J]. Advanced Energy Materials, 2023, 13(23): 2300149. DOI: 10.1002/aenm.202300149. |
| 16 | CHEN S Q, WU C, SHEN L F, et al. Challenges and perspectives for NASICON-type electrode materials for advanced sodium-ion batteries[J]. Advanced Materials, 2017, 29(48): 1700431. DOI: 10.1002/adma.201700431. |
| 17 | AHSAN Z, CAI Z F, WANG S, et al. Recent development of phosphate based polyanion cathode materials for sodium-ion batteries[J]. Advanced Energy Materials, 2024, 14(27): 2400373. DOI: 10.1002/aenm.202400373. |
| 18 | LIU Q N, HU Z, CHEN M Z, et al. The cathode choice for commercialization of sodium-ion batteries: Layered transition metal oxides versus Prussian blue analogs[J]. Advanced Functional Materials, 2020, 30(14): 1909530. DOI: 10.1002/adfm. 201909530. |
| 19 | WANG D, DENG Y P, LIU Y H, et al. Sodium-ion batteries towards practical application through gradient Mn-based layer-tunnel cathode[J]. Nano Energy, 2023, 110: 108340. DOI: 10.1016/j.nanoen.2023.108340. |
| 20 | RAJAGOPALAN R, TANG Y G, JIA C K, et al. Understanding the sodium storage mechanisms of organic electrodes in sodium ion batteries: Issues and solutions[J]. Energy & Environmental Science, 2020, 13(6): 1568-1592. DOI: 10.1039/C9EE03637G. |
| 21 | HUANG J Q, DU R, ZHANG H, et al. Low-cost Prussian blue analogues for sodium-ion batteries and other metal-ion batteries[J]. Chemical Communications, 2023, 59(61): 9320-9335. DOI: 10.1039/d3cc01548c. |
| 22 | VERMA V, KUMAR S, MANALASTAS W Jr, et al. Progress in rechargeable aqueous zinc-and aluminum-ion battery electrodes: Challenges and outlook[J]. Advanced Sustainable Systems, 2019, 3(1): 1800111. DOI: 10.1002/adsu.201800111. |
| 23 | LIU Y A, LIU H Q, ZHANG R Z, et al. Recent progress of manganese-based Prussian blue analogue cathode materials for sodium-ion batteries[J]. Ionics, 2024, 30(1): 39-59. DOI: 10.1007/s11581-023-05295-2. |
| 24 | PENG J, ZHANG W, LIU Q N, et al. Prussian blue analogues for sodium-ion batteries: Past, present, and future[J]. Advanced Materials, 2022, 34(15): e2108384. DOI: 10.1002/adma.202108384. |
| 25 | ZHOU A J, CHENG W J, WANG W, et al. Hexacyanoferrate-type Prussian blue analogs: Principles and advances toward high-performance sodium and potassium ion batteries[J]. Advanced Energy Materials, 2021, 11(2): 2000943. DOI: 10.1002/aenm. 202000943. |
| 26 | XIE B X, SUN B Y, GAO T Y, et al. Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries[J]. Coordination Chemistry Reviews, 2022, 460: 214478. DOI: 10.1016/j.ccr.2022.214478. |
| 27 | LI W J, HAN C, CHENG G, et al. Chemical properties, structural properties, and energy storage applications of Prussian blue analogues[J]. Small, 2019, 15(32): e1900470. DOI: 10.1002/smll.201900470. |
| 28 | SUN J G, YE H L, OH J A S, et al. Elevating the discharge plateau of Prussian blue analogs through low-spin Fe redox induced intercalation pseudocapacitance[J]. Energy Storage Materials, 2021, 43: 182-189. DOI: 10.1016/j.ensm.2021.09.004. |
| 29 | KUMAR A, YUSUF S, KELLER L. Structural and magnetic properties of Fe [Fe(CN)6] 4H2O[J]. Physical Review B, 2005, 71: 054414. |
| 30 | KIM D, HWANG T, LIM J M, et al. Hexacyanometallates for sodium-ion batteries: Insights into higher redox potentials using d electronic spin configurations[J]. Physical Chemistry Chemical Physics, 2017, 19(16): 10443-10452. DOI: 10.1039/C7CP00378A. |
| 31 | HURLBUTT K, WHEELER S, CAPONE I, et al. Prussian blue analogs as battery materials[J]. Joule, 2018, 2(10): 1950-1960. DOI: 10.1016/j.joule.2018.07.017. |
| 32 | WANG B Q, HAN Y, WANG X, et al. Prussian blue analogs for rechargeable batteries[J]. iScience, 2018, 3: 110-133. DOI:10.1016/j.isci.2018.04.008. |
| 33 | QIAN J F, WU C, CAO Y L, et al. Prussian blue cathode materials for sodium-ion batteries and other ion batteries[J]. Advanced Energy Materials, 2018, 8(17): 1702619. DOI: 10.1002/aenm. 201702619. |
| 34 | GEBERT F, CORTIE D L, BOUWER J C, et al. Epitaxial nickel ferrocyanide stabilizes jahn-teller distortions of manganese ferrocyanide for sodium-ion batteries[J]. Angewandte Chemie (International Ed), 2021, 60(34): 18519-18526. DOI: 10.1002/anie.202106240. |
| 35 | WU X Y, DENG W W, QIAN J F, et al. Single-crystal FeFe(CN)6 nanoparticles: A high capacity and high rate cathode for Na-ion batteries[J]. Journal of Materials Chemistry A, 2013, 1(35): 10130-10134. DOI: 10.1039/C3TA12036H. |
| 36 | CHEN M Z, ZHANG Y Y, XING G C, et al. Building high power density of sodium-ion batteries: Importance of multidimensional diffusion pathways in cathode materials[J]. Frontiers in Chemistry, 2020, 8: 152. DOI: 10.3389/fchem.2020.00152. |
| 37 | NORDSTRAND J, TOLEDO-CARRILLO E, VAFAKHAH S, et al. Ladder mechanisms of ion transport in Prussian blue analogues[J]. ACS Applied Materials & Interfaces, 2022, 14(1): 1102-1113. DOI: 10.1021/acsami.1c20910. |
| 38 | GE L N, SONG Y J, NIU P C, et al. Elaborating the crystal water of Prussian blue for outstanding performance of sodium ion batteries[J]. ACS Nano, 2024, 18(4): 3542-3552. DOI: 10.1021/acsnano.3c11169. |
| 39 | XIAO Y, XIAO J, ZHAO H K, et al. Prussian blue analogues for sodium-ion battery cathodes: A review of mechanistic insights, current challenges, and future pathways[J]. Small, 2024, 20(35): e2401957. DOI: 10.1002/smll.202401957. |
| 40 | QIAO Y, WEI G Y, CUI J B, et al. Prussian blue coupling with zinc oxide as a protective layer: An efficient cathode for high-rate sodium-ion batteries[J]. Chemical Communications, 2019, 55(4): 549-552. DOI: 10.1039/C8CC07951J. |
| 41 | CHEN J S, WEI L, MAHMOOD A, et al. Prussian blue, its analogues and their derived materials for electrochemical energy storage and conversion[J]. Energy Storage Materials, 2020, 25: 585-612. DOI: 10.1016/j.ensm.2019.09.024. |
| 42 | PENG J, ZHANG B, HUA W B, et al. A disordered rubik's cube-inspired framework for sodium-ion batteries with ultralong cycle lifespan[J]. Angewandte Chemie (International Ed), 2023, 62(6): e202215865. DOI: 10.1002/anie.202215865. |
| 43 | WU X Y, RU Y, BAI Y, et al. PBA composites and their derivatives in energy and environmental applications[J]. Coordination Chemistry Reviews, 2022, 451: 214260. DOI: 10.1016/j.ccr. 2021.214260. |
| 44 | ZHANG H, GAO Y, PENG J, et al. Prussian blue analogues with optimized crystal plane orientation and low crystal defects toward 450 wh kg-1 alkali-ion batteries[J]. Angewandte Chemie (International Ed), 2023, 62(27): e202303953. DOI: 10.1002/anie.202303953. |
| 45 | SONG J, WANG L, LU Y H, et al. Removal of interstitial H2O in hexacyanometallates for a superior cathode of a sodium-ion battery[J]. Journal of the American Chemical Society, 2015, 137(7): 2658-2664. DOI: 10.1021/ja512383b. |
| 46 | NAI J W, LOU X W D. Hollow structures based on Prussian blue and its analogs for electrochemical energy storage and conversion[J]. Advanced Materials, 2019, 31(38): e1706825. DOI: 10.1002/adma.201706825. |
| 47 | GAO Y T, HUANG Y, PAN H G, et al. Towards defect-free Prussian blue-based battery electrodes[J]. Journal of Alloys and Compounds, 2023, 950: 169886. DOI: 10.1016/j.jallcom. 2023.169886. |
| 48 | WANG P Y, LI Y H, ZHU D G, et al. Treatment dependent sodium-rich Prussian blue as a cathode material for sodium-ion batteries[J]. Dalton Transactions, 2022, 51(25): 9622-9626. DOI: 10.1039/d2dt01171a. |
| 49 | 陈娜, 李安琪, 郭子祥, 等. 钠离子电池普鲁士蓝材料结构构建及优化的研究进展 [J]. 储能科学与技术. 2023, 12(11): 3340-3351. |
| CHEN N, LI A Q, GUO Z X, et al. Research progress on the construction and optimization of Prussian blue material structure for sodium-ion batteries. Energy Storage Science and Technology, 2023, 12(11), 3340-3351. | |
| 50 | YAN X M, YANG Y, LIU E S, et al. Improved cycling performance of Prussian blue cathode for sodium ion batteries by controlling operation voltage range[J]. Electrochimica Acta, 2017, 225: 235-242. DOI: 10.1016/j.electacta.2016.12.121. |
| 51 | YU S L, LI Y, LU Y H, et al. A promising cathode material of sodium iron–nickel hexacyanoferrate for sodium ion batteries[J]. Journal of Power Sources, 2015, 275: 45-49. DOI: 10.1016/j.jpowsour.2014.10.196. |
| 52 | CHEN Z Y, FU X Y, ZHANG L L, et al. High-performance Fe-based Prussian blue cathode material for enhancing the activity of low-spin Fe by Cu doping[J]. ACS Applied Materials & Interfaces, 2022, 14(4): 5506-5513. DOI: 10.1021/acsami.1c23793. |
| 53 | LI L, NIE P, CHEN Y B, et al. Novel acetic acid induced Na-rich Prussian blue nanocubes with iron defects as cathodes for sodium ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(19): 12134-12144. DOI: 10.1039/C9TA01965K. |
| 54 | REN W H, QIN M S, ZHU Z X, et al. Activation of sodium storage sites in Prussian blue analogues via surface etching[J]. Nano Letters, 2017, 17(8): 4713-4718. DOI: 10.1021/acs.nanolett.7b01366. |
| 55 | OH G, KIM J, KANSARA S, et al. Experimental and computational optimization of Prussian blue analogues as high-performance cathodes for sodium-ion batteries: A review[J]. Journal of Energy Chemistry, 2024, 93: 627-662. DOI: 10.1016/j.jechem.2024.02.013. |
| 56 | PENG J, WANG J S, YI H C, et al. A dual-insertion type sodium-ion full cell based on high-quality ternary-metal Prussian blue analogs[J]. Advanced Energy Materials, 2018, 8(11): 1702856. DOI: 10.1002/aenm.201702856. |
| 57 | WU X Y, SHAO M M, WU C H, et al. Low defect FeFe(CN)6 framework as stable host material for high performance Li-ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(36): 23706-23712. DOI: 10.1021/acsami.6b06880. |
| 58 | QIN M S, REN W H, MENG J S, et al. Realizing superior Prussian blue positive electrode for potassium storage via ultrathin nanosheet assembly[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(13): 11564-11570. DOI: 10.1021/acssuschemeng.9b01454. |
| 59 | PENG J, GAO Y, ZHANG H, et al. Ball milling solid-state synthesis of highly crystalline Prussian blue analogue Na2- xMnFe(CN)6 cathodes for all-climate sodium-ion batteries[J]. Angewandte Chemie (International Ed), 2022, 61(32): e202205867. DOI: 10.1002/anie.202205867. |
| 60 | CHEN R J, HUANG Y X, XIE M, et al. Chemical inhibition method to synthesize highly crystalline Prussian blue analogs for sodium-ion battery cathodes[J]. ACS Applied Materials & Interfaces, 2016, 8(46): 31669-31676. DOI: 10.1021/acsami.6b10884. |
| 61 | ZUO D X, WANG C P, WU J W, et al. Effect of co-precipitation pH on the electrochemical properties of Prussian blue electrode materials for sodium-ion batteries[J]. Solid State Ionics, 2019, 336: 120-128. DOI: 10.1016/j.ssi.2019.03.014. |
| 62 | ZHOU A J, XU Z M, GAO H C, et al. Size-, water-, and defect-regulated potassium manganese hexacyanoferrate with superior cycling stability and rate capability for low-cost sodium-ion batteries[J]. Small, 2019, 15(42): e1902420. DOI: 10.1002/smll.201902420. |
| 63 | CHEN J, HUANG K L, LIU S Q. Insoluble metal hexacyanoferrates as supercapacitor electrodes[J]. Electrochemistry Communications, 2008, 10(12): 1851-1855. DOI:10.1016/j.elecom.2008.07.046. |
| 64 | QIAN J F, ZHOU M, CAO Y L, et al. NaxMyFe(CN)6(M=Fe, co, Ni): A new class of cathode materials for sodium ion batteries[J]. Journal of Electrochemistry, 2012, 18(2): 108-112. DOI: 10.61558/2993-074x.2888 |
| 65 | SHEN Z L, GUO S H, LIU C L, et al. Na-rich Prussian white cathodes for long-life sodium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 16121-16129. DOI: 10.1021/acssuschemeng.8b02758. |
| 66 | WANG L, SONG J, QIAO R M, et al. Rhombohedral Prussian white as cathode for rechargeable sodium-ion batteries[J]. Journal of the American Chemical Society, 2015, 137(7): 2548-2554. DOI: 10.1021/ja510347s. |
| 67 | WANG L, LU Y H, LIU J, et al. A superior low-cost cathode for a Na-ion battery[J]. Angewandte Chemie (International Ed), 2013, 52(7): 1964-1967. DOI: 10.1002/anie.201206854. |
| 68 | HUANG Y X, XIE M, WANG Z H, et al. A chemical precipitation method preparing hollow-core-shell heterostructures based on the Prussian blue analogs as cathode for sodium-ion batteries[J]. Small, 2018. 14(28): 1801246. DOI: 10.1002/smll.201801246. |
| 69 | XIANG J J, HAO Y C, GAO Y T, et al. Tailoring the growth of iron hexacyanoferrates for high-performance cathode of sodium-ion batteries[J]. Journal of Alloys and Compounds, 2023, 946: 169284. DOI: 10.1016/j.jallcom.2023.169284. |
| 70 | CHEN Z Y, ZHANG L L, FU X Y, et al. Synergistic modification of Fe-based Prussian blue cathode material based on structural regulation and surface engineering[J]. ACS Applied Materials & Interfaces, 2022, 14(38): 43308-43318. DOI: 10.1021/acsami. 2c11823. |
| 71 | YAN C X, ZHAO A L, ZHONG F P, et al. A low-defect and Na-enriched Prussian blue lattice with ultralong cycle life for sodium-ion battery cathode[J]. Electrochimica Acta, 2020, 332: 135533. DOI: 10.1016/j.electacta.2019.135533. |
| 72 | XU Y, CHANG M, FANG C, et al. In situ FTIR-assisted synthesis of nickel hexacyanoferrate cathodes for long-life sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(33): 29985-29992. DOI: 10.1021/acsami.9b10312. |
| 73 | XU Y, WAN J, HUANG L, et al. Structure distortion induced monoclinic nickel hexacyanoferrate as high-performance cathode for Na-ion batteries[J]. Advanced Energy Materials, 2019, 9(4): 1803158. DOI: 10.1002/aenm.201803158. |
| 74 | PENG F W, YU L, GAO P Y, et al. Highly crystalline sodium manganese ferrocyanide microcubes for advanced sodium ion battery cathodes[J]. Journal of Materials Chemistry A, 2019, 7(39): 22248-22256. DOI: 10.1039/C9TA08603J. |
| 75 | CHEN W C, LI S J, XU H Y, et al. Effect of particle dispersion on electrochemical performance of Prussian blue analogues electrode materials for sodium ion batteries[J]. Chemphyschem, 2024, 25(5): e202300960. DOI: 10.1002/cphc.202300960. |
| 76 | HUANG T B, DU G Y, QI Y R, et al. A Prussian blue analogue as a long-life cathode for liquid-state and solid-state sodium-ion batteries[J]. Inorganic Chemistry Frontiers, 2020, 7(20): 3938-3944. DOI: 10.1039/D0QI00872A. |
| 77 | XIE B X, WANG L G, SHU J, et al. Understanding the structural evolution and lattice water movement for rhombohedral nickel hexacyanoferrate upon sodium migration[J]. ACS Applied Materials & Interfaces, 2019, 11(50): 46705-46713. DOI: 10.1021/acsami.9b15073. |
| 78 | LIU J H, WANG Y C, JIANG N, et al. Vacancies-regulated Prussian blue analogues through precipitation conversion for cathodes in sodium-ion batteries with energy densities over 500 wh/kg[J]. Angewandte Chemie (International Ed), 2024, 63(39): e202400214. DOI: 10.1002/anie.202400214. |
| 79 | UR REHMAN W, JIANG Z Y, QU Z, et al. Highly crystalline Prussian blue cubes filled with tin oxide as anode materials for lithium-ion batteries[J]. Applied Surface Science, 2022, 604: 154533. DOI: 10.1016/j.apsusc.2022.154533. |
| 80 | YOU Y, WU X L, YIN Y X, et al. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries[J]. Energy & Environmental Science, 2014, 7(5): 1643-1647. DOI: 10.1039/C3EE44004D. |
| 81 | LIU Y, WEI G Y, MA M Y, et al. Role of acid in tailoring Prussian blue as cathode for high-performance sodium-ion battery[J]. Chemistry, 2017, 23(63): 15991-15996. DOI: 10.1002/chem.201703081. |
| 82 | MING H, TORAD N L K, CHIANG Y D, et al. Size- and shape-controlled synthesis of Prussian Blue nanoparticles by a polyvinylpyrrolidone-assisted crystallization process[J]. CrystEng Comm, 2012, 14(10): 3387-3396. DOI: 10.1039/C2CE25040C. |
| 83 | LIU Y J, FAN S W, GAO Y, et al. Isostructural synthesis of iron-based Prussian blue analogs for sodium-ion batteries[J]. Small, 2023, 19(43): e2302687. DOI: 10.1002/smll.202302687. |
| 84 | WANG P Y, ZHU D G, LI Y H, et al. Buffer solution induced highly crystalline sodium-rich Prussian blue for sodium storage[J]. Chemical Communications, 2024, 60(12): 1603-1606. DOI: 10.1039/D3CC06123J. |
| 85 | REN W H, ZHU Z X, QIN M S, et al. Prussian white hierarchical nanotubes with surface-controlled charge storage for sodium-ion batteries[J]. Advanced Functional Materials, 2019, 29(15): 1806405. DOI: 10.1002/adfm.201806405. |
| 86 | XU C M, PENG J, LIU X H, et al. Na1.51Fe [Fe(CN)6]0.87·1.83H2O hollow nanospheres via non-aqueous ball-milling route to achieve high initial coulombic efficiency and high rate capability in sodium-ion batteries[J]. Small Methods, 2022, 6(8): 2200404. DOI: 10.1002/smtd.202200404. |
| 87 | ZHANG P, XU C L, ZHAO J M, et al. Rapid and solvent-free mechanochemical synthesis of Na iron hexacyanoferrate for high-performance Na-ion batteries[J]. Materials Today Energy, 2022, 27: 101027. DOI: 10.1016/j.mtener.2022.101027. |
| 88 | TANG W, XIE Y Y, PENG F W, et al. Electrochemical performance of NaFeFe(CN)6 prepared by solid reaction for sodium ion batteries[J]. Journal of the Electrochemical Society, 2018, 165(16): A3910-A3917. DOI: 10.1149/2.0701816jes. |
| 89 | LUO Y, PENG J Y, YIN S M, et al. Acid-assisted ball mill synthesis of carboxyl-functional-group-modified Prussian blue as sodium-ion battery cathode[J]. Nanomaterials, 2022, 12(8): 1290. DOI: 10.3390/nano12081290. |
| 90 | GONG W Z, WAN M, ZENG R, et al. Ultrafine Prussian blue as a high-rate and long-life sodium-ion battery cathode[J]. Energy Technology, 2019, 7(7): 1900108. DOI: 10.1002/ente.201900108. |
| 91 | LUCERO M, ARMITAGE D B, YANG X, et al. Ball milling-enabled Fe2.4+ to Fe3+ redox reaction in Prussian blue materials for long-life aqueous sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2023, 15(30): 36366-36372. DOI: 10.1021/acsami. 3c07304. |
| 92 | YANG J H, WANG H S, LU L H, et al. Large-scale synthesis of berlin green Fe[Fe(CN)6] microcubic crystals[J]. Crystal Growth & Design, 2006, 6(11): 2438-2440. DOI: 10.1021/cg060469r. |
| 93 | WANG M M, TAO Y M, ZHANG D Y, et al. High rate and cyclic performance of Na3–2 xMgxV2(PO4)3/C cathode for sodium-ion batteries[J]. Journal of Materials Science: Materials in Electronics, 2020, 31(21): 18360-18369. DOI: 10.1007/s10854-020-04381-9. |
| 94 | BAGGIO B F, VICENTE C, PELEGRINI S, et al. Morphology and structure of electrodeposited Prussian blue and Prussian white thin films[J]. Materials, 2019, 12(7): 1103. DOI: 10.3390/ma12071103. |
| 95 | LAMPRECHT X, ZELLNER P, YESILBAS G, et al. Fast-charging capability of thin-film Prussian blue analogue electrodes for aqueous sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2023, 15(19): 23951-23962. DOI: 10.1021/acsami.3c02633. |
| 96 | 李林, 朱登贵, 孙淑敏, 等. 普鲁士蓝及其类似物作为钠离子电池正极材料的研究进展[J]. 分子科学学报, 2023, 39(1): 1-10. DOI: 10.13563/j.cnki.jmolsci.2022.06.005. |
| LI L, ZHU D G, SUN S M, et al. Research progress of Prussian blue and its analogues as cathode materials for sodium ion batteries[J]. Journal of Molecular Science, 2023, 39(1): 1-10. DOI: 10.13563/j.cnki.jmolsci.2022.06.005. | |
| 97 | 陈强, 李敏, 李敬发. 普鲁士蓝类似物及其衍生物在钾离子电池中的应用[J]. 储能科学与技术, 2021, 10(3): 1002-1015. DOI: 10.19799/j.cnki.2095-4239.2021.0029. |
| CHEN Q, LI M, LI J F. Application of Prussian blue analogs and their derivatives in potassium ion batteries[J]. Energy Storage Science and Technology, 2021, 10(3): 1002-1015. DOI: 10.19799/j.cnki.2095-4239.2021.0029. | |
| 98 | TANG X, LIU H, SU D W, et al. Hierarchical sodium-rich Prussian blue hollow nanospheres as high-performance cathode for sodium-ion batteries[J]. Nano Research, 2018, 11(8): 3979-3990. DOI: 10.1007/s12274-018-1979-y. |
| 99 | FENG J X, TONG Y X, LI G R. Epitaxial growth modulation of hollow topologies for high-performance electrocatalysts[J]. Chem, 2018, 4(9): 2015-2017. DOI: 10.1016/j.chempr.2018.08.021. |
| 100 | YOU Y, YU X Q, YIN Y X, et al. Sodium iron hexacyanoferrate with high Na content as a Na-rich cathode material for Na-ion batteries[J]. Nano Research, 2015, 8(1): 117-128. DOI: 10.1007/s12274-014-0588-7. |
| 101 | HUANG Y, ZHANG X, JI L, et al. Boosting the sodium storage performance of Prussian blue analogs by single-crystal and high-entropy approach[J]. Energy Storage Materials, 2023, 58: 1-8. DOI: 10.1016/j.ensm.2023.03.011. |
| 102 | YANG D Z, XU J, LIAO X Z, et al. Structure optimization of Prussian blue analogue cathode materials for advanced sodium ion batteries[J]. Chemical Communications, 2014, 50(87): 13377-13380. DOI: 10.1039/c4cc05830e. |
| 103 | XU Z, SUN Y, XIE J, et al. High-performance Ni/Fe-codoped manganese hexacyanoferrate by scale-up synthesis for practical Na-ion batteries[J]. Materials Today Sustainability, 2022, 18: 100113. DOI: 10.1016/j.mtsust.2022.100113. |
| 104 | XI Y M, LU Y C. Electrochemically active Mn-doped iron hexacyanoferrate as the cathode material in sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(34): 39022-39030. DOI: 10.1021/acsami.2c07779. |
| 105 | XIE M, XU M H, HUANG Y X, et al. Na2NixCo1- xFe(CN)6: A class of Prussian blue analogs with transition metal elements as cathode materials for sodium ion batteries[J]. Electrochemistry Communications, 2015, 59: 91-94. DOI: 10.1016/j.elecom. 2015.07.014. |
| 106 | GAO P, CHEN Z, GONG Y X, et al. The role of cation vacancies in electrode materials for enhanced electrochemical energy storage: Synthesis, advanced characterization, and fundamentals[J]. Advanced Energy Materials, 2020, 10(14): 1903780. DOI: 10.1002/aenm.201903780. |
| 107 | LIU J C, LIU J, TANG M X, et al. Boosting sodium storage in Prussian blue analogs through iron vacancies and copper doping[J]. Advanced Functional Materials, 2024, 34(17): 2314167. DOI: 10.1002/adfm.202314167. |
| 108 | ZHANG H, PENG J, LI L, et al. Low-cost zinc substitution of iron-based Prussian blue analogs as long lifespan cathode materials for fast charging sodium-ion batteries[J]. Advanced Functional Materials, 2023, 33(2): 2210725. DOI: 10.1002/adfm.202210725. |
| 109 | 朱子翼, 董鹏, 张举峰, 等. 新一代储能钠离子电池正极材料的改性研究进展[J]. 化工进展, 2020, 39(3): 1043-1056. DOI: 10.16085/j.issn.1000-6613.2019-0840. |
| ZHU Z Y, DONG P, ZHANG J F, et al. Research progress on modification of cathode materials for new generation energy storage sodium-ion batteries[J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1043-1056. DOI: 10.16085/j.issn.1000-6613.2019-0840. | |
| 110 | TANG Y, ZHANG W X, XUE L H, et al. Polypyrrole-promoted superior cyclability and rate capability of NaxFe[Fe(CN)6] cathodes for sodium-ion batteries[J]. Journal of Materials Chemistry A, 2016, 4(16): 6036-6041. DOI: 10.1039/C6TA00876C. |
| 111 | ZHANG Q, FU L, LUAN J Y, et al. Surface engineering induced core-shell Prussian blue@polyaniline nanocubes as a high-rate and long-life sodium-ion battery cathode[J]. Journal of Power Sources, 2018, 395: 305-313. DOI: 10.1016/j.jpowsour.2018.05.085. |
| 112 | LI X, SUN X L. Interface design and development of coating materials in lithium-sulfur batteries[J]. Advanced Functional Materials, 2018, 28(30): 1801323. DOI: 10.1002/adfm. 201801323. |
| 113 | 赵毅伟, 张福华, 颜顺, 等. 普鲁士蓝类钠离子电池正极材料导电性研究进展[J]. 储能科学与技术, 2024, 13(5): 1474-1486. DOI: 10.19799/j.cnki.2095-4239.2023.0895 |
| ZHAO Y W, ZHANG F H, YAN S, et al. Research progress on the conductivity of Prussian blue sodium-ion battery cathode materials[J]. Energy Storage Science and Technology, 2024, 13(5): 1474-1486. DOI: 10.19799/j.cnki.2095-4239.2023.0895 | |
| 114 | WAN M, TANG Y, WANG L L, et al. Core-shell hexacyanoferrate for superior Na-ion batteries[J]. Journal of Power Sources, 2016, 329: 290-296. DOI: 10.1016/j.jpowsour. 2016.08.059. |
| 115 | JIANG Y Z, YU S L, WANG B Q, et al. Prussian Blue@C composite as an ultrahigh-rate and long-life sodium-ion battery cathode[J]. Advanced Functional Materials, 2016, 26(29): 5315-5321. DOI: 10.1002/adfm.201600747. |
| 116 | CHEN Y C, WOO H J, RIZWAN M, et al. Nanoscale morphology control of Na-rich Prussian blue cathode materials for sodium ion batteries with good thermal stability[J]. ACS Applied Energy Materials, 2019, 2(12): 8570-8579. DOI: 10.1021/acsaem.9b01491. |
| 117 | ZHU P, WANG Y P, LI J, et al. Continuous production of high-capacity iron-based Prussian blue sodium-ion cathode materials using a rotor-stator spinning disk reactor[J]. ACS Applied Energy Materials, 2023, 6(11): 6141-6150. DOI: 10.1021/acsaem.3c00679. |
| 118 | ZHANG R Z, LIU Y A, LIU H Q, et al. Y-tube assisted coprecipitation synthesis of iron-based Prussian blue analogues cathode materials for sodium-ion batteries[J]. RSC Advances, 2024, 14(17): 12096-12106. DOI: 10.1039/d4ra00762j. |
| 119 | BAJI D S, NAIR S, SANTHANAGOPALAN D. Chemical reduction of Prussian blue nanocubes to obtain alkali ion containing cathodes and their battery applications[J]. Sustainable Energy & Fuels, 2022, 6(7): 1719-1726. DOI: 10.1039/D2SE00171C. |
| 120 | WU E A, BANERJEE S, TANG H M, et al. A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries[J]. Nature Communications, 2021, 12(1): 1256. DOI: 10.1038/s41467-021-21488-7. |
| 121 | HUANG Y X, ZHAO L Z, LI L, et al. Electrolytes and electrolyte/electrode interfaces in sodium-ion batteries: From scientific research to practical application[J]. Advanced Materials, 2019, 31(21): e1808393. DOI: 10.1002/adma.201808393. |
| 122 | LI C C, XU H Y, NI L, et al. Nonaqueous liquid electrolytes for sodium-ion batteries: Fundamentals, progress and perspectives[J]. Advanced Energy Materials, 2023, 13(40): 2301758. DOI: 10.1002/aenm.202301758. |
| 123 | TIAN Z N, ZOU Y G, LIU G, et al. Electrolyte solvation structure design for sodium ion batteries[J]. Advanced Science, 2022, 9(22): e2201207. DOI: 10.1002/advs.202201207. |
| 124 | LIN Z H, XIA Q B, WANG W L, et al. Recent research progresses in ether- and ester-based electrolytes for sodium-ion batteries[J]. InfoMat, 2019, 1(3): 376-389. DOI: 10.1002/inf2.12023. |
| 125 | WU F, ZHU N, BAI Y, et al. Highly safe ionic liquid electrolytes for sodium-ion battery: Wide electrochemical window and good thermal stability[J]. ACS Applied Materials & Interfaces, 2016, 8(33): 21381-21386. DOI: 10.1021/acsami.6b07054. |
| 126 | XU K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries[J]. Chemical Reviews, 2004, 104(10): 4303-4417. DOI: 10.1021/cr030203g. |
| 127 | PIERNAS-MUÑOZ M J, CASTILLO-MARTÍNEZ E, GÓMEZ-CÁMER J L, et al. Optimizing the electrolyte and binder composition for Sodium Prussian Blue, Na1- xFex+(1/3)(CN)6·yH2O, as cathode in sodium ion batteries[J]. Electrochimica Acta, 2016, 200: 123-130. DOI: 10.1016/j.electacta.2016.02.188. |
| 128 | VIET THIEU Q Q, HOANG H, LE V T, et al. Enhancing electrochemical performance of sodium Prussian blue cathodes for sodium-ion batteries via optimizing alkyl carbonate electrolytes[J]. Ceramics International, 2021, 47(21): 30164-30171. DOI: 10.1016/j.ceramint.2021.07.195. |
| 129 | CAMACHO P S, WERNERT R, DUTTINE M, et al. Impact of synthesis conditions in Na-rich Prussian blue analogues[J]. ACS Applied Materials & Interfaces, 2021, 13(36): 42682-42692. DOI: 10.1021/acsami.1c09378. |
| 130 | SYED MOHD FADZIL S A F, WOO H J, AZZAHARI A D, et al. Sodium-rich Prussian blue analogue coated by poly(3, 4-ethylenedioxythiophene) polystyrene sulfonate as superior cathode for sodium-ion batteries[J]. Materials Today Chemistry, 2023, 30: 101540. DOI: 10.1016/j.mtchem.2023.101540. |
| 131 | CHUN J Y, WANG X L, WEI C L, et al. Flexible and free-supporting Prussian blue analogs/MXene film for high-performance sodium-ion batteries[J]. Journal of Power Sources, 2023, 576: 233165. DOI: 10.1016/j.jpowsour.2023.233165. |
| 132 | YUAN T, FU X P, WANG Y, et al. Enhanced conductivity and stability of Prussian blue cathodes in sodium-ion batteries by surface vapor-phase molecular self-assembly[J]. Nano Research, 2024, 17(5): 4221-4230. DOI: 10.1007/s12274-023-6394-3. |
| 133 | TANG Y, WANG L, HU J W, et al. Epitaxial nucleation of NaxFeFe(CN)6@rGO with improved lattice regularity as ultrahigh-rate cathode for sodium-ion batteries[J]. Advanced Energy Materials, 2024, 14(7): 2303015. DOI: 10.1002/aenm.202303015. |
| 134 | CHEN R J, HUANG Y X, XIE M, et al. Preparation of Prussian blue submicron particles with a pore structure by two-step optimization for Na-ion battery cathodes[J]. ACS Applied Materials & Interfaces, 2016, 8(25): 16078-16086. DOI: 10.1021/acsami.6b04151. |
| 135 | ZUO D X, WANG C P, HAN J J, et al. Oriented formation of a Prussian blue nanoflower as a high performance cathode for sodium ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(43): 16229-16240. DOI: 10.1021/acssuschemeng.0c05466. |
| 136 | SONG B Y, XIONG X S, PENG Y, et al. Review of electrolyte additives for secondary sodium batteries[J]. Advanced Energy Materials, 2024, 14(30): 2401407. DOI: 10.1002/aenm. 202401407. |
| 137 | SHAO T L, LI C, LIU C Y, et al. Electrolyte regulation enhances the stability of Prussian blue analogues in aqueous Na-ion storage[J]. Journal of Materials Chemistry A, 2019, 7(4): 1749-1755. DOI: 10.1039/C8TA10860A. |
| [1] | Yi LIANG, Tao WEI, Guangda YIN, Dequan HUANG. Design of a lithiophilic Ag-3D-Cu electrode and its electrochemical performance [J]. Energy Storage Science and Technology, 2025, 14(2): 515-524. |
| [2] | Yangfeng WANG, Jiaao HOU, Zichen ZHU, Cong SUO, Shuandi HOU. Research progress on hard-carbon closed-pore structure of sodium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(2): 555-569. |
| [3] | Yonggang CHANG, Jinhao ZHANG, Wei XIE, Xiuchun LI, Yilin WANG, Chengmeng CHEN. Capacity enhancement strategy of hard carbon anode for sodium-ion battery: A review [J]. Energy Storage Science and Technology, 2025, 14(2): 544-554. |
| [4] | Xunchang JIANG, Kelin YU, Daxiang YANG, Minhui LIAO, Yang ZHOU. Preparation of PDOL-based solid electrolyte by in-situ polymerization and its application in lithium metal batteries [J]. Energy Storage Science and Technology, 2025, 14(1): 1-12. |
| [5] | Yijie YAO, Junwei ZHANG, Yanjun ZHAO, Hongcheng LIANG, Dongni ZHAO. Effect of interfacial dynamics on low temperature performance of sodium-ion batteries [J]. Energy Storage Science and Technology, 2025, 14(1): 30-41. |
| [6] | Dingbang HAO, Yongli LI. Na0.85Ni0.3Fe0.2Mn0.5O1.95F0.05@CuO cathode materials for high-rate and long cycling stability sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(8): 2489-2498. |
| [7] | Yinan HE, Kai ZHANG, Junwu ZHOU, Xinyang WANG, Bailin ZHENG. Influence of external loads on the cycling performance of silicon anode lithium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(8): 2559-2569. |
| [8] | Yuan YAO, Ruoqi ZONG, Jianli GAI. Research progress of antimony- and bismuth-based metallic anode materials for sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(8): 2649-2664. |
| [9] | Weiqi LIN, Qiaoyu LU, Yuhong CHEN, Linyuan QIU, Yurong JI, Lianyu GUAN, Xiang DING. Advances in cathode materials for low-temperature sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(7): 2348-2360. |
| [10] | Renchao FENG, Yu DONG, Xinyu ZHU, Cai LIU, Sheng CHEN, Da LI, Ruoyu GUO, Bin WANG, Jionghui WANG, Ning LI, Yuefeng SU, Feng WU. Research progress on graphite oxide-based anodes for sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(6): 1835-1848. |
| [11] | Cong SUO, Yangfeng WANG, Zichen ZHU, Yan YANG. Research progress of soft carbon as negative electrodes in sodium-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(6): 1807-1823. |
| [12] | Wanrui LI, Wenjun LI, Xiaoqing WANG, Shengli LU, Xilian XU. Research progress of manganese/vanadium-based oxide heterostructure cathodes for zinc-ion batteries [J]. Energy Storage Science and Technology, 2024, 13(5): 1496-1515. |
| [13] | Yinbao MIAO, Wenhua ZHANG, Weihao LIU, Shuai WANG, Zhe CHEN, Wang PENG, Jie ZENG. Preparation and performance of lithium-rich cathode material Li1.2Ni0.13Co0.13Mn0.54O2 [J]. Energy Storage Science and Technology, 2024, 13(5): 1427-1434. |
| [14] | Xin LIU, Xiling MAO, Xinyu YAN, Junqiang WANG, Mengwei LI. Preparation and electrochemical properties of NiMn-MOF with 3D pore network electrode materials [J]. Energy Storage Science and Technology, 2024, 13(2): 361-369. |
| [15] | Yang ZHOU, Peiyu HAN, Yingchun NIU, Chunming XU, Quan XU. Fabrication of metal-organic framework-derived C-Bi/CC electrode materials and their electrochemical properties in ICRFB [J]. Energy Storage Science and Technology, 2024, 13(2): 381-389. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
